Sulfonation of Benzene: Sulfonation is the introduction of a sulpho group, -SO2OH into an organic compound. Various sulfonating agents like, concentrated H2SO4, oleum, sulphur trioxide, sulphur dioxide and oxygen, sulphurous acid in the form of alkali salts, sulphur dioxide and chlorine, chlorosulphonic acid, etc. may be used to carry out sulphonation.
Polycyclic aromatic hydrocarbons (like anthracene, and phenanthrene) are sulfonated most easily. Naphthalene is sulfonated with difficulty and benzene, with even greater difficulty.
The sulfonation of benzene is often carried out with either concentrated H2SO4 or with fuming H2SO4 (i.e, sulphuric acid containing sulphur trioxide, SO3). Sulphur trioxide is the effective electrophile involved in the sulphonation reaction. The rate of reaction depends on the concentrations of the aromatic ring and sulphur trioxide and not on sulphuric acid. It is a reversible reaction and proceeds through the following mechanism.
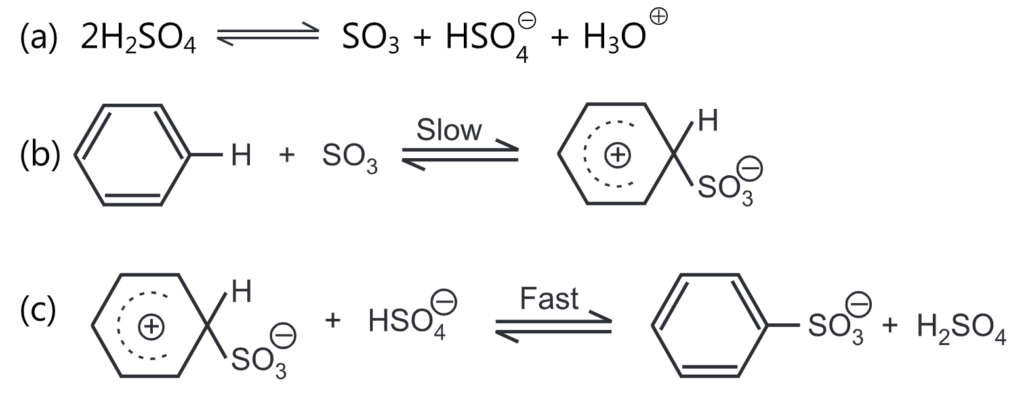
The water separated in the reaction reduces the concentration of sulphuric acid, which loses its sulfonating properties, and causes a reversible reaction, i.e., the hydrolysis of the sulphonic acid being formed. The reversibility of the reaction is observed when the product is treated with steam which results in the replacement of the -SO3H group by ‘H’.
The readiness with which sulphonation takes place depends upon the nature of substituents present on the aromatic ring. Electron-releasing substituents increase the ease of sulphonation reaction in the following order.
OH > OR > NH2> NHCOR > R
Electron withdrawing substituents and halogens hinder the introduction of the sulpho group.
Temperature condition is the critical factor in sulphonation reaction. Higher temperature not only accelerates the process but also promotes the formation of by-products which include sulphones, polysulphone acids and oxidation and condensation products. The temperature condition also determines the position of attack for sulpho group in the aromatic ring. For example, at low temperatures, α-naphthalene sulphonic acid is formed several times faster than the ẞ-isomer. In some cases, the catalyst also governs the site of attack of the sulpho group. For example, ẞ-anthraquinone sulphonic acid is mainly formed when anthraquinone is sulfonated in absence of a catalyst while α-anthraquinone sulphonic acid is formed when it is sulfonated in presence of mercury salts.
Make sure you also check our other amazing Article on : Nitration on Benzene