A critical point is a particular temperature and pressure at which two phases (like liquid and vapor) can co-exist. The substance above the critical temperature and pressure or above the critical point is known as a supercritical fluid.
The property of the supercritical fluid is in between gas and pure liquid so that they are also known as dense gases or compressible liquid. Supercritical fluid has good solvation power, low viscosity, higher diffusibility, and good penetrating power. With the help of the supercritical fluid extraction technique, we can separate the constituents from the drug with the help of supercritical fluid. Generally, we can separate the constituents from the solid matrix but it may also be liquid. CO2 is one of the most common supercritical fluids. To make CO2 supercritical fluid the critical temperature is 31°C and the critical pressure is 74 bars.
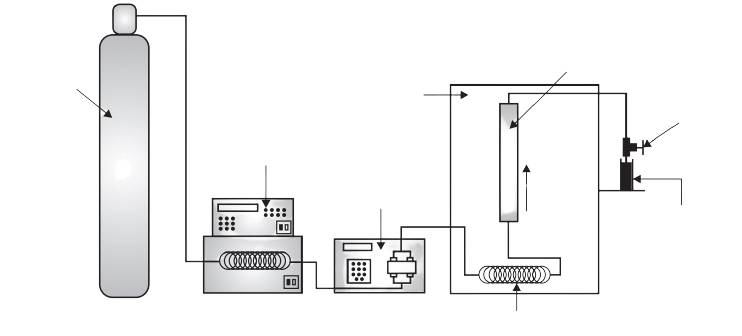
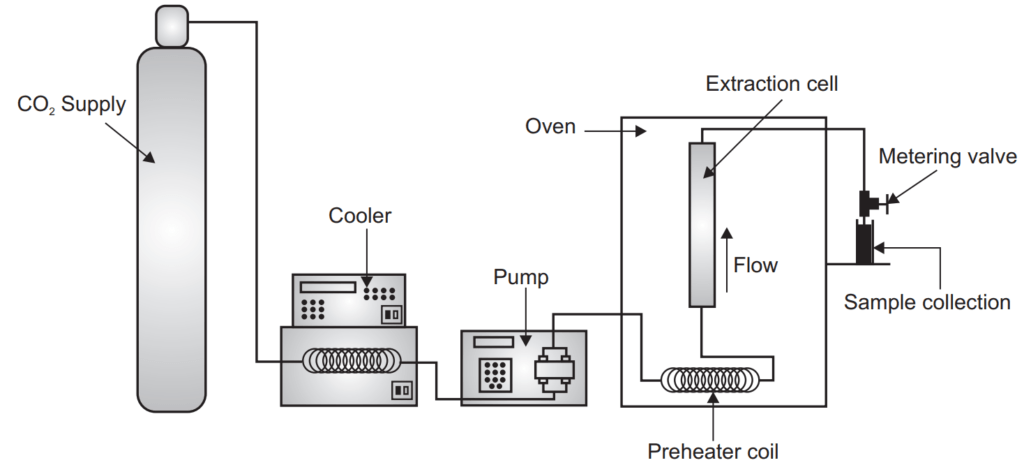
Supercritical fluid extraction instrumentation
The different component in the supercritical fluid extraction is the first one is a fluid reservoir which contains the fluid-like gas cylinder in the case of CO2. Another one is the pump; the pump may be a syringe pump or reciprocating pump. Another important is the extractor which may be made of stainless steel and can stand to high pressure like 300 to 600 atmosphere. Another important component of the supercritical fluid extractor is the restrictor. It is important for the controlled and systematic release of the pressure inside the extractor vesicle. The restrictor may be a fixed type or variable type. The isolated constituent is collected in a collector where it can be detected with the help of a detector. Sometimes modifiers are also added into the supercritical fluid to increase its versatility like one to ten percent of methanol is added in CO2.
During the process the liquid is pumped into a heating zone and heated to supercritical temperature then the supercritical fluid passes through the extraction vessel where it diffuses to the solid matrix and dissolves the active constituents. The extracted material with supercritical fluid comes into the separator or collector unit where the pressure is low. The extracted material settles down here and the CO2 can then be cooled, recycled, or discharge into the environment. The isolated constituent can be detected with the help of a detector.
Advantages and Limitations of the Supercritical fluid extraction
- The supercritical fluid extraction technique is very rapid and there is no need for organic solvent for the extraction purpose.
- The thermolabile substance can also be extracted with this technique.
- It is a versatile and efficient continuous method with complete separation and recovery of the solvent.
- Sometimes its consistency and reproducibility may vary.
- It is an expensive technique because it needs high pressure which increases its cost.
- CO2 cannot always be used as a solvent as it is non-polar and so limited dissolving power particularly in the case of a polar solvent.
Make sure you also check our other amazing Article on : Percolation Extraction