Thiophene Reactions: Thiophene is slightly more nucleophilic than benzene. The negative charge is more accumulated on C2 – and C5 – atoms. While sulfur bears a positive charge. Hence, it easily undergoes electrophilic substitution preferably at C2 – and C5 – positions.
Chemical Reactions of Thiophene
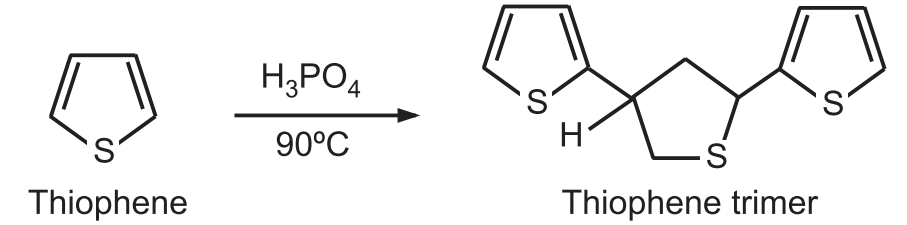
1. Protonation: Thiophene is very stable in the action of acids. Very strong acids like the action of hot phosphoric acid give thiophene trimer.
2. Oxidation: Thiophene ring is stable to the action of various oxidizing agents. However, the side chains can be oxidized to carboxylic acid groups. When heated with nitric acid, the ring breaks down to maleic acid and oxalic acid.
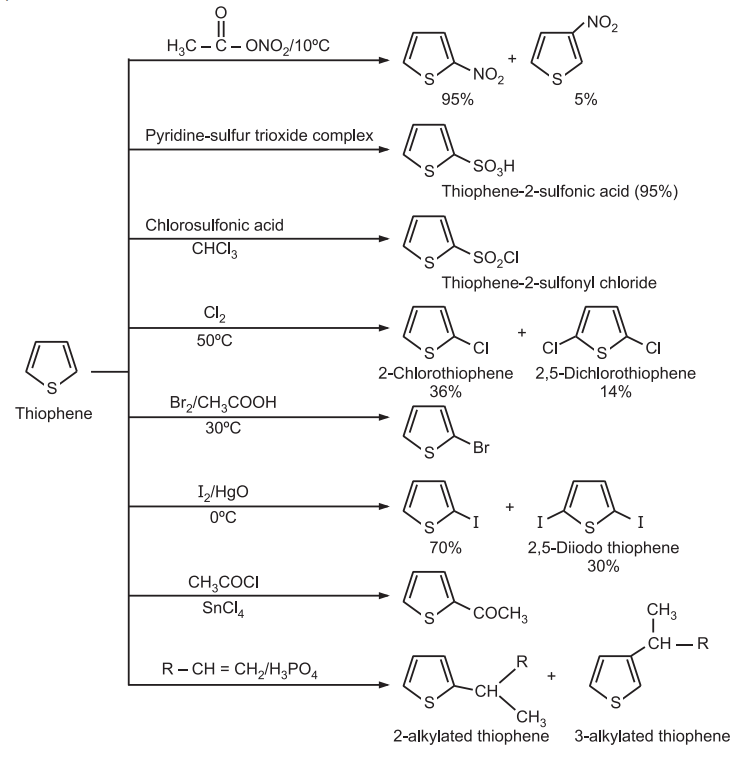
3. Electrophilic Substitution: The reactivity order for electrophilic substitution reaction is: pyrrole > furan > thiophene >benzene. The preferred site of the attack in thiophene is the C2-position.
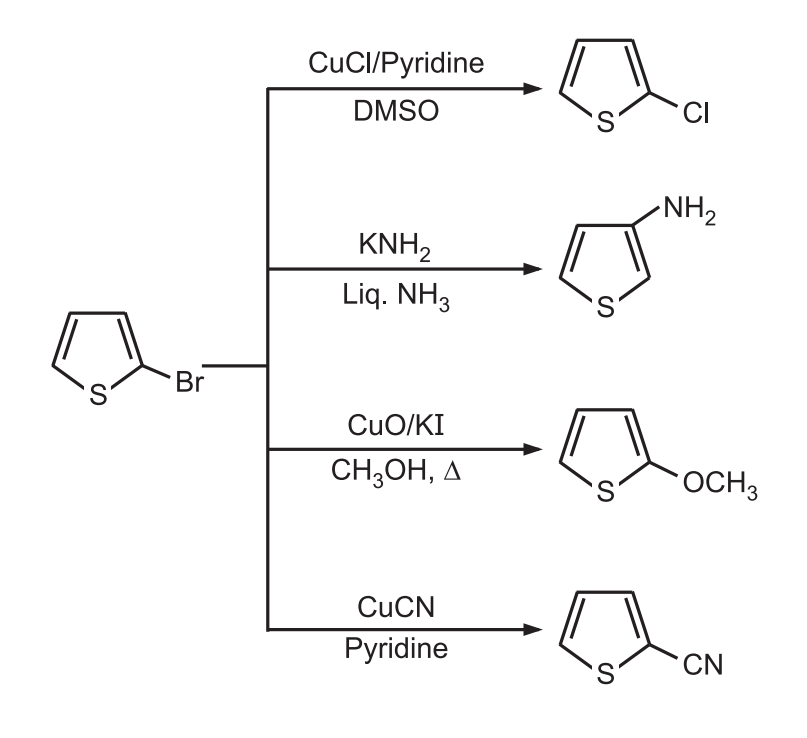
4. Nucleophilic Substitution: Thiophene substituted with electron-withdrawing substituents is much more reactive to nucleophilic substitution.
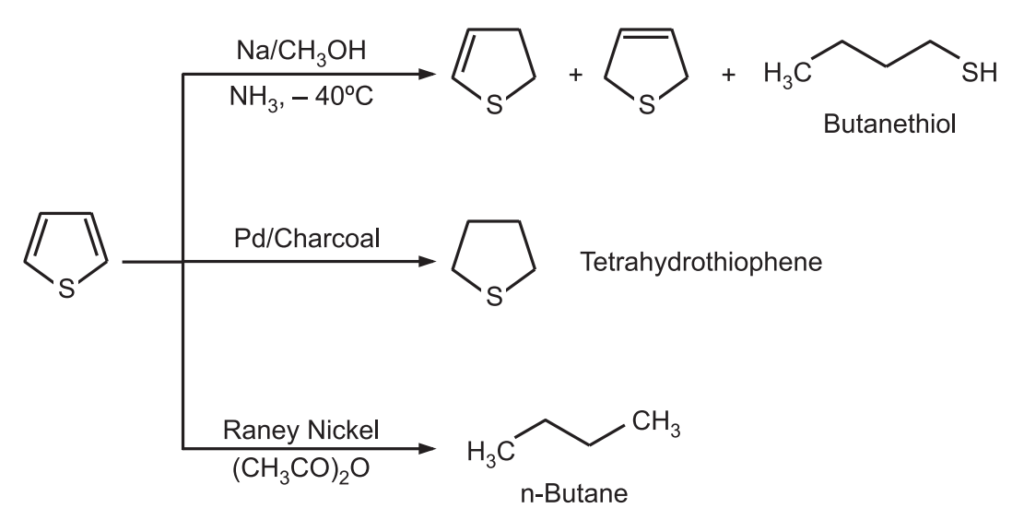
5. Reduction:
Applications in Drug (Thiophene) Synthesis
Thiophene derivatives possess remarkable activities like antibacterial, anti-inflammatory, anti-anxiety, anti-psychotic, anti-arrhythmic, and anticancer. Examples include lornoxicam (thiophene analog of piroxicam), pyrantel (anti-parasitic), raltitrexed (anticancer), cephalothin (antimicrobial), suproprofen (anti-inflammatory), ticrynafen (anti-hypertensive), clotiazepam (anti-anxiety), ticlopidine (platelet aggregation inhibitor), etc.
Make sure you also check our other amazing Article on : Thiophene Synthesis