Plant tissue culture is a general term to culture the isolated plant organs (particularly of isolated roots but, to a lesser extent of stem tips, immature embryo, leaf primordia, flower structures, and even the cells and the protoplasts) under an aseptic environment. The plant growth and development occur in two different ways namely determinate growth where plant growth occurs with certain shapes and sizes like leaves, fruits, flowers etc. Types of plant tissue cultures depend on the type of explant used. Hence, plant tissue culture is broadly classified into eight types viz
- Seed Culture
- Embryo Culture
- Meristem Culture
- Bud Culture
- Callus Culture
- Cell Suspension Culture
- Anther Culture
- Protoplast Culture
- Hairy root culture
- Immobilized cell culture
Table of Contents
Seed culture
Seeds are cultured in-vitro to generate seedlings or plants in aseptic conditions for raising the sterile seedling. The seed culture is done to get the different kinds of explants from aseptically grown plants that help in better maintenance of aseptic tissue (Fig. 1.1).
Importance
- Increasing efficiency of germination of seeds.
- It is possible to be independent of symbiotic germination.
- It is important in the production of Orchids.
Embryo culture
Embryo culture is the sterile isolation and growth of an immature or mature embryo in-vitro for procurement of a viable plant. Embryo developed (initially white in colour) from wide hybridization between two different species may not mature fully due to embryo[1]endosperm incompatibility. The whole process is known as Embryogenesis (Fig. 1.2).
Importance
- It is useful in the production of haploids.
- It helps in the prevention of seed dormancy.
- It helps in the shortening of the breeding cycle.
- It helps in the prevention of embryo abortion with early ripening stone fruits.
Meristem Culture
It is the culture technique by which the apical meristem of shoots of angiosperms and gymnosperms are cultured to get the disease-free plants. Generally meristem tips, between 0.2-0.5 mm, most frequently produce virus-free plants and this method is referred to as meristem-tip culture (Fig. 1.3). These explants are cultured on a medium containing cytokinin (plant hormone).

Importance
- It helps in the production of virus-free plants.
- It helps in Germplasm conservation.
- It helps in the production of transgenic plants.
- It helps in rapid clonal multiplication.
- The method is more successful in the case of herbaceous plants than woody plants.
- It helps in the culture of Potato, Banana, Cardamom, Sugar cane, sweet potato etc.
Bud culture
Buds contain active meristems in the leaf axils, which are capable of growing into a shoot. Each node of the stem is cut and allowed to grow on a nutrient media to develop the shoot tip from the axil which ultimately develops into a new plantlet. In the axillary bud method, where the axillary buds are isolated from the leaf axils and develop into shoot tips under little high cytokinin concentration (Fig. 3.4).
Importance
- Easy step for micropropagation.
- Easy method for production of disease-free plants.
- Isolation of phytoconstituents is easy.
Callus Culture
It is a culture of an undifferentiated mass of parenchyma cell produced from an explant of a seedling or other plant part in agar medium under aseptic condition is known as callus culture. A callus is densely aggregated, uncontrolled, undifferentiated, unorganized, aerated homogenous parenchymatous mass (Fig. 1.5).

Importance
- It is the source of tissue for plant regeneration. The whole plant can be regenerated in large numbers from callus tissue through manipulation of the nutrient and plant hormones in the culture medium. This phenomenon is known as plant regeneration or organogenesis or morphogenesis.
- Chromosomal variation occurs genetically or epigenetically in the cells of callus tissue.
- An increased amount of secondary metabolites are obtained by extraction of the particular callus tissue.
- This method is the source of Tissue for Cell Suspension Culture.
- Several biochemical assays are performed from callus culture.
Principles:
- Aseptic preparation of plant material.
- Selection of suitable nutrient medium supplemented with the appropriate ratio of plant growth regulators such as auxins and cytokinins or only appropriate auxin.
- Incubation of culture under controlled physical conditions.
Steps involved in Callus culture:
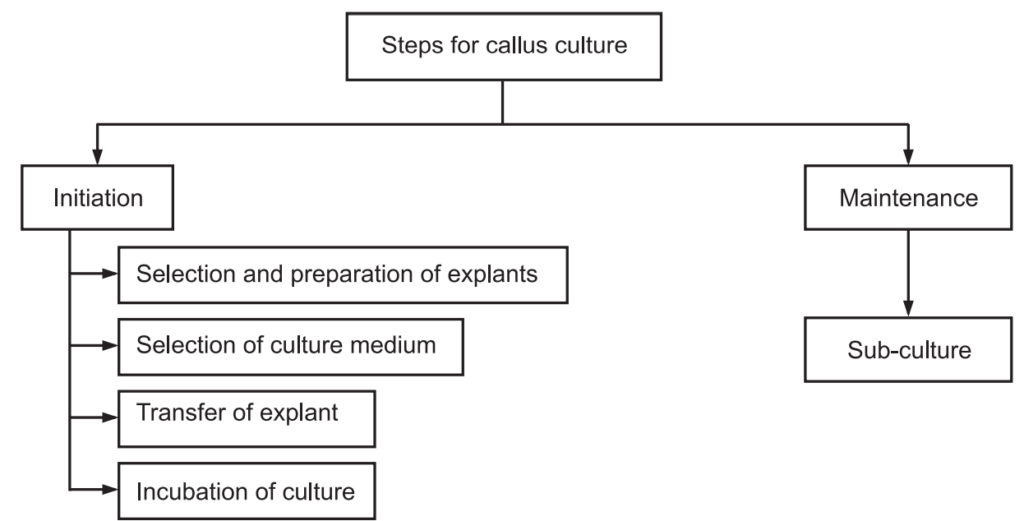
Preparation of explants: Based on the totipotency of the explants, they are selected in young parts. After selection, they are surface sterilized with the help of various steps such as washing under running tap water for several times, 1% batiste in treatment, followed by 70% alcohol, 1% sodium hypochlorite, 0.1% mercuric chloride and finally cleaned with double distilled water.
Selection of culture medium: Plant growth hormones such as auxin and cytokinin in the ratio of 10: 1 to 100: 1 induces roots whereas 1: 10 to 1: 100 induces shoots. Furthermore 1: 1 ratio helps in callus growth. Mainly solidified agar gel containing MS medium is favourable for callus culture. Media are autoclaved at 15 psi pressure for 15-20 mins at 121°C. The pH is adjusted to 5.7 using 0.1 M HCl or NaOH. Generally, agar is used at 0.8 to 1.0% concentration and widely used gelling agent. Agar is resistant to enzymatic hydrolysis at incubation temperature and also neutral to media constituents hence do not react with any other ingredients. Due to these properties, agar is used as solidifying media.
Transfer of explant: Sterilized explants are aseptically transferred media containing agar nutrients with the help of sterile forceps in a laminar airflow cabinet.
Incubation of culture: Explant containing culture vessels are aseptically incubated under controlled physical conditions viz. temperature is to maintain 25 ± 2°C, photoperiod should maintain 16 hours a day and 8 hours dark conditions, light intensity 2000 to 3000 Lux, relative humidity 55% to 60%. Generally, a BOD incubator is used for incubation. During this period callus is formed through three stages likely, induction (where metabolic activities of the cell increase), cell division (where active cells are divided) and cell differentiation (where secondary metabolites are formed through morphological and physiological differentiation).
BOD incubator: It is the most versatile and reliable low-temperature incubator which is designed to maintain at 20°C for Biological Oxygen Demand or Biochemical Oxygen Demand (BOD) determination. It provides controlled temperature conditions for accelerated tests and exposures. It is based on the principle of thermo-electricity. The incubator has a thermostat that maintains a constant temperature by creating a thermal gradient.
Conditions:
- Temperature: Standard temperature range: 2°C to 50°C.
- Temperature control: ± 0.5°C, 24 hours light and temperature programming. Automatic digital temperature recorder.
- Light: Adjustable fluorescent light up to 10,000 Lux.
- Relative humidity: 20 to 98%. Relative humidity control: ± 3%, uniform air circulation.
- Attached with a shaker for agitation in suspension culture.
Maintenance: Maintenance of callus is possible by sub-culturing callus in a fresh medium. This is a vital stage for the further growth of the Callus because in the same medium nutrient and water content are reduced as a result metabolic toxins are accumulated. Hence, sub-culturing is necessary for the fresh medium which is repeated after every 4-5 weeks. During the growth, period callus appears as yellowish, white, green, or pigmented with brown and purple. Pigmentation is sometimes uniform throughout the callus or some regions remain unpigmented. If callus grown is dark, due to lack of chlorophyll it showed white, if grown in light, then callus formed as green, if carotenoid pigments are more, then callus formed as yellow, purple pigmentation of callus indicated the formation of Anthocyanins whereas more phenolic content indicates the callus is brown in colour.
Cell Suspension Culture
Suspension culture is defined as a uniform suspension of separate cells in a liquid medium where agar is not used. In this medium, callus fragments are transferred to a liquid medium and agitated continuously to keep the cell alive and separate. Agitation is also achieved by a rotary shaker attached within the BOD incubator at a rate of 50 to 160 rpm. This culture technique is used to study the morphological and biochemical changes during their growth and developmental phases.
Importance
- Suspension culture is consists of only single cells which are physiologically and biochemically uniform.
- This culture is capable of contributing significant information about cell physiology, biochemistry, metabolic events etc.
- It is important for plant biotransformation and plant genetic engineering.
- No toxic products are formed with this culture technique.
- It helps for induction in somatic embryos and shoots.
- It helps in in-vitro mutagenesis and the selection of mutants.
- It helps in the production of secondary metabolites.
- Shorter duration and continuous process.
Anther Culture
It is the in-vitro culture technique of anther containing microspores from unopened flower bud or immature pollen grains (Pollen culture) on a suitable nutrient medium under aseptic conditions for the purpose of development of haploid plantlets. By this culture technique haploid cells are obtained which is known as Androgenesis (Fig. 1.7).
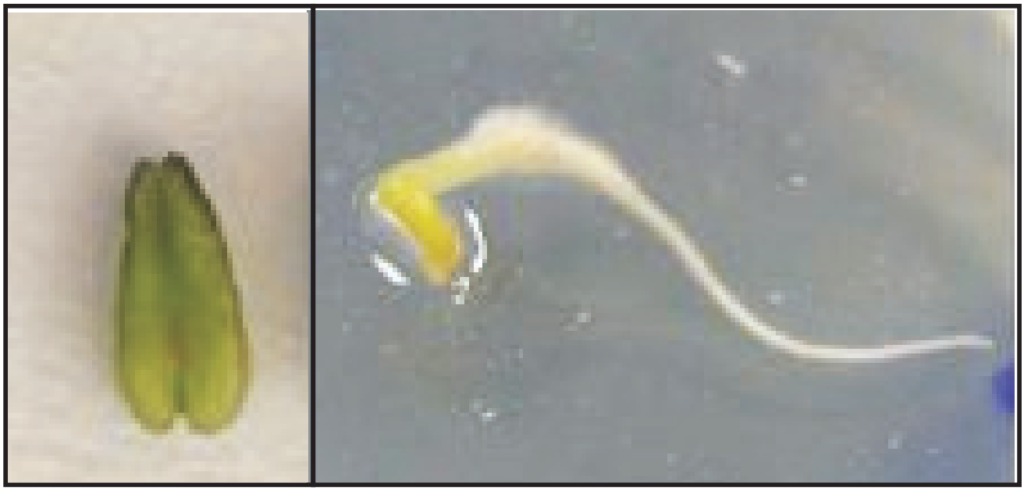
Steps:
The young flower buds are collected and washed under running tap water. Surface sterilized by immersing in 70% ethanol or 2.0% sodium hypochlorite solution. Then washed in sterile water and transferred into a sterile Petri dish. With the help of a sterile sharp scalpel, the buds are split open and other lobes are taken out. One of the anther lobes of each bud is checked by crushing into acetocarmine stain under the microscope for the proper stage of microspore development. The filament portions are removed from the selected anther lobes. The intact anther lobes are placed into proper media and then incubated at 24°-28°C in dark for 3-8 weeks. The haploid embryos or plantlets develop, come out by bursting the anther lobes.
Importance
- It is used to study genetic recombination in higher plants.
- It is used to study the mode of differentiation from a single cell to whole organisms.
- It is used to study factors controlling pollen embryogenesis of higher plants.
- It is used for mutation studies.
- It is used for hybrid development.
- It is used for genome mapping.
- It is used for the formation of double haploids that are homozygous and fertile.
Protoplast Culture
In this culture method, isolated protoplasts are cultured either in a liquid medium or semisolid agar medium in a thin layer or as small drops of nutrient medium in a sterile Petri dish. Protoplast cells are having cell membranes without cell walls (Fig. 1.8). They are isolated either by the enzymatic method or by the mechanical method. Mechanical method is manual method and there is a possibility of a loss of protoplast cell due to friction force in motor and pestle. This method is avoided by following the technique likely a small piece of epidermis is selected from the explants then the cells are subjected to plasmolysis. This causes protoplasts shrinking and finally, the tissue is dissected to release the protoplasts from the cell wall. In the enzymatic method, enzymes such as cellulase, hemicellulase and pectinase are used. At first, pectinase is used to breaks up the cell aggregates into individual cells by degrading middle lamella then these free cells are exposed to cellulase to release protoplasts from the cell wall. This enzymatic method is used widely to get more yields of protoplast cells as well as minimal or less damage to the protoplast cells.
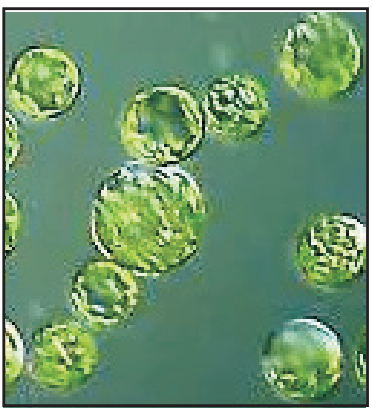
Methods of culture
Protoplast culture is carried out in liquid culture, agar culture, droplet culture, co-culture, hanging droplet culture, immobilised/bead culture and feeder layer technique. In liquid culture, protoplasts easily get divided in liquid media and the osmotic pressure of the medium is regulated. In agar culture, agarose is most frequently used to solidify protoplast culture media. Protoplast suspension is taken at double density and mixed with melted agar medium at 45°C and mixed well and plated in a small Petri dish. In the droplet culture technique, suspending protoplasts in liquid culture media are placed on Petri dishes in the form of a droplet, the cultured protoplasts clump together at the centre of droplets. In the co-culture technique, the newly isolated protoplast suspension is mixed with an authentic fast-growing protoplast suspension and mixed protoplasts are plated for the development of the isolated protoplast. By hanging drop technique, protoplasts are cultured in an inverted droplet on the inner surface of the lid of the Petri dish. In bead culture, the protoplasts suspension is mixed with polymers such as alginate, carrageenan, etc. and then small beads are made by dripping into the liquid medium and then cultured into a liquid medium. In the Feeder layer technique, protoplast cell suspensions are exposed to X-rays (to inhibit cell division) and then plating them in a thin layer on agar plates for the isolated less number of protoplasts.
Importance of Protoplast Culture:
- This technique is used to study Morphogenesis.
- This technique is used to study Photosynthesis.
- It helps in gene transfer.
- It helps in the study of cell wall formation and their osmotic behaviour.
- It helps in crop improvement through somatic hybridization.
- Protoplast cells also can regenerate into whole plants.
- It develops novel hybrid plants through protoplast fusion.
Hairy Root Culture
This technique is also known as transformed root culture. It is a culture produced after the infection of explants or cultures by a naturally occurring soil bacterium Agrobacterium rhizogenes that contains root-inducing plasmids (Ri plasmids) are infect plant roots and cause them to produce an opine (food source for the bacterium) and abnormally very fast growth. It grows by increasing the rate of cell division and cell elongation. Hairy roots are induced in most of the dicot plants by genetic transformation (through T-DNA, transfer DNA) with A. rhizogenes. Hairy root culture shows a very high growth rate in the absence of the growth regulator and does not require conditioning of the medium (Fig. 1.9).
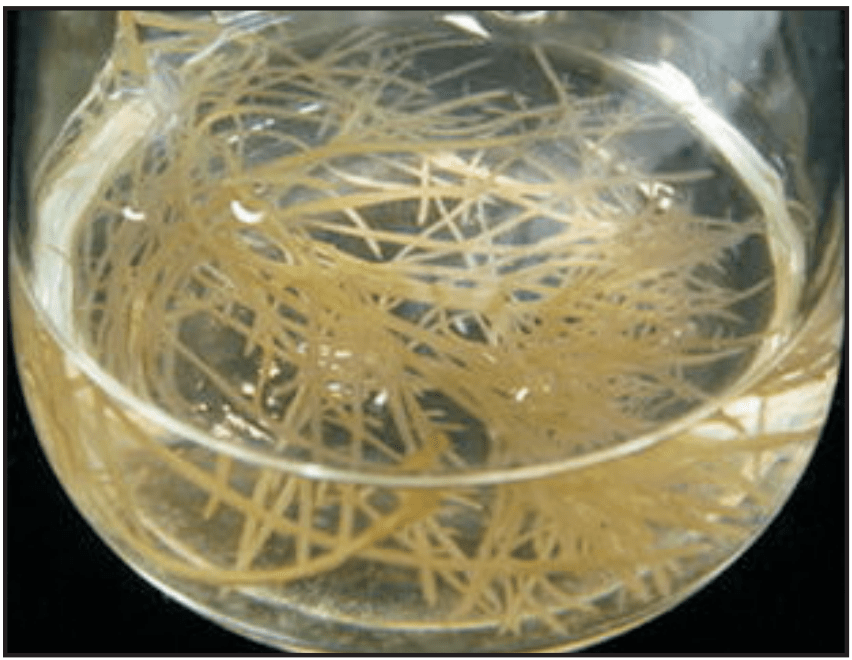
Importance
- It helps in the production of high secondary metabolites.
- The culture grows under phytohormone free conditions.
- The culture shows fast growth that reduces culture time and easy handling.
- It helps in the functional analysis of genes.
- It is also used for the regeneration of whole plants.
- The culture expresses foreign proteins.
- The culture is genetically and biosynthetically stable.
Immobilized Cell Culture
It is the technique that confines the cells to a definite region in space, known as a matrix. During that condition, the catalytic activity of the cells retains and prevents their entry into the mobile phase. Immobilization is achieved by binding these cells onto or within solid support. Some of the important materials are used as matrix-like gelatin, polylysine, agarose, alginate etc.
The cells are immobilized by covalently, adsorbed, cross-linked, encapsulated and entrapment methods (Fig. 1.10).
Importance
- The encapsulation method protects cells from mechanical damage in large fermenters.
- It is used in synthetic seed technology.
- It is used for the transfer of protoplast.
- It is cultured as a single cell for a longer period.
- It helps in the conservation of rare cells for further growth into the whole plant.
- It helps in the production of a higher amount of plant secondary metabolites.
- It helps in biotransformation.
Application of Plant Tissue Culture
Plant tissue culture technology has been used in almost all the fields of biosciences. The desirable products produced by plant tissue cultures are as diversified as is the industry itself. Its applications include:
- Development of Clonal and Micro-Propagation through the multiplication of genetically identical plants or crops. It is very used in horticulture, in the crops which have long seed dormancy, tree species, orchids, and many fruit plants.
- Production of biomass energy which is useful in forestry.
- Increased production of secondary metabolites helps in the isolation of the constituents for drug discovery as well as various pharmaceutical formulations.
- Procurement of Genetic Variability is due to cells of various ploidy levels and genetic constitution of the initial explant or also may be developed due to different cultural conditions. It helps for the production of hybrid plants by polyploidy, mutation, and hybridization techniques. It is a great application in the field of horticulture as well as in Agronomy. It helps in the development of useful characteristics such as resistance to a particular disease, herbicide resistance, and stress tolerance.
- Somatic Embryogenesis and Synthetic Seed development by which the mass production of adventitious embryos occurs that ultimately develop into complete plantlet in maturing media. This method is useful for the production of disease-free propagules as well as the production of asexually propagating plants. It is an innovative technique in the agriculture field.
- Breaking Dormancy is further challenging for hard-coated seeds. By this culture technique, the seed dormancy period is reduced and the breeding cycle is shortened which improves the early growth of plants.
- Propagation of haploid Plants through anther or pollen culture (androgenesis) or through ovaries or ovule culture (gynogenesis).
- Establishment of Somatic Hybrids through Somatic hybridization, i.e., the asexual hybridization. It is carried out by the fusion of protoplast cells by hybridization. Products of fusion between two protoplasts (heterokaryon) are cultured to regenerate a new somatic hybrid plant of the desired genotype.
- Development of Transgenic Plants by carrying genes for different traits like insect resistance, herbicide tolerance, delayed ripening, increased amino acid and vitamin content, improved oil quality, etc.
- Germplasm Conservation through the in-vitro method is gaining importance. The storage collection provides a cost-effective alternative to growing plants under field conditions, nurseries, or greenhouses. Recently, pollen preservation is very important for endangered plant species.
Make sure you also check our other amazing Article on : Conservation Of Medicinal Plants
