Different blood groups are associated with genetically determined differences in antigens on the surface of the membrane of RBCs and antibodies in blood serum. There are two main systems used to classify blood donated for administration by transfusion. If the donor’s blood does not match with that of the recipient, the incompatibility results in agglutination and lysis of donated red cells after transfusion. The agglutinated cells block capillaries and the products of lysis when in excessive amounts, damaging the kidney tubules. The resultant condition is serious and can cause death.
Table of Contents
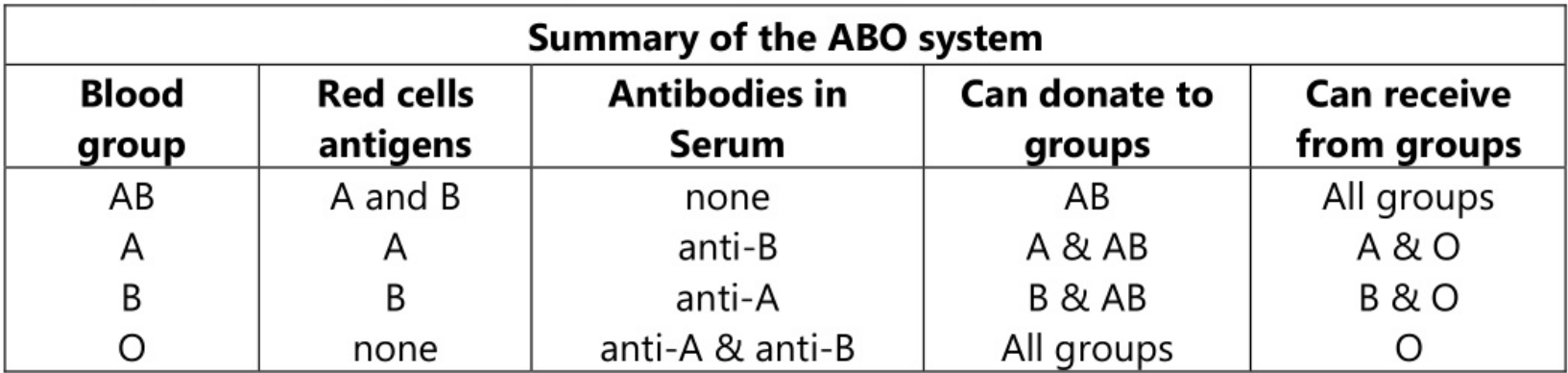
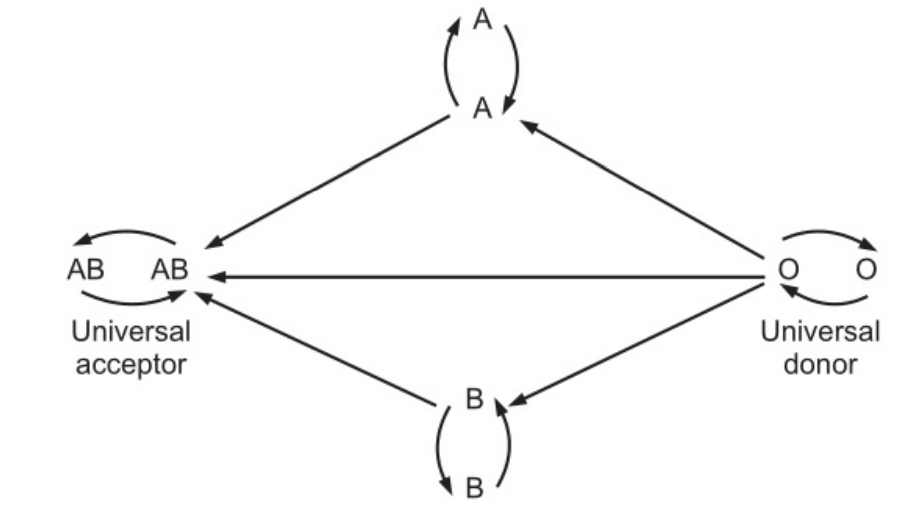
ABO System
In some people, there are genetically determined antigens on the surface of the membrane of RBCs and natural antibodies in serum. The antibodies are inherited and are not associated with acquired immunity. The summary of the ABO system is presented as follows.
Rhesus System
In over 80 per cent of people the Rhesus factor is present on the membrane of red cells i.e. they are rhesus positive (Rh+ve). The Rhesus factor consists of a number of antigens of which D is the most common. Individuals with antigen are classified as Rh+ve. Administration of Rh+ve blood to Rh-ve recipients stimulates an immune response with the production of antibodies that cause haemolysis of the transfused red cells. Second and subsequent encounters with Rh+ve red cells lead to a sharp increase in antibody production.
Erythroblastosis Foetal
The most common problem with Rh incompatibility may arise during pregnancy. Normally, there is no direct contact between maternal and foetal blood when a woman is pregnant. However, if a small amount of Rh+ve blood leaks from the foetus through the placenta into the bloodstream of the Rh-ve mother, the mother will start to make anti-Rh antibodies. Since the greatest possibility of foetal blood transfer occurs at delivery, the newborn baby will be affected. If the mother becomes pregnant again, her anti-Rh antibodies can cross the placenta and enter the bloodstream of the foetus. If the foetus is Rh-ve, there is no problem, because Rh-ve blood does not have Rh antigen. If the foetus is Rh+ve, haemolysis may occur in foetal blood. The haemolysis brought about on by foetal-maternal incompatibility is called as Erythroblastosis foetal. This condition is prevented by giving all Rh-ve mothers an injection of anti-Rh antibodies called anti-Rh y globulin (Rheogram) soon after every delivery, miscarriage, or abortion.
Leucocytes or White Blood Cells (WBCs)
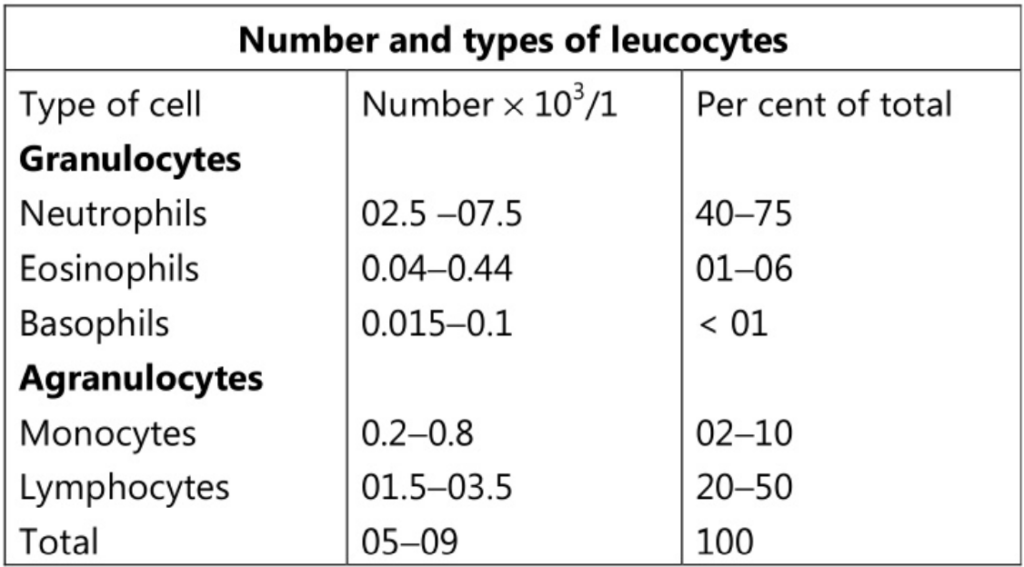
These cells have an important function in defending the body against microbes and other foreign materials. They are the largest blood cells and account for about 01 per cent of the blood volume. They contain nuclei and some of them have granules in their cytoplasm. They are of two main types:
- Granulocytes (Polymorphonuclear leucocytes): Neutrophils, Eosinophils and Basophils.
- Agranulocytes: Monocytes and Lymphocytes.
Granulocytes
Their formation is termed as granulopoiesis. During development, they follow a common line of development through myeloblast to myelocyte before differentiating into three types.
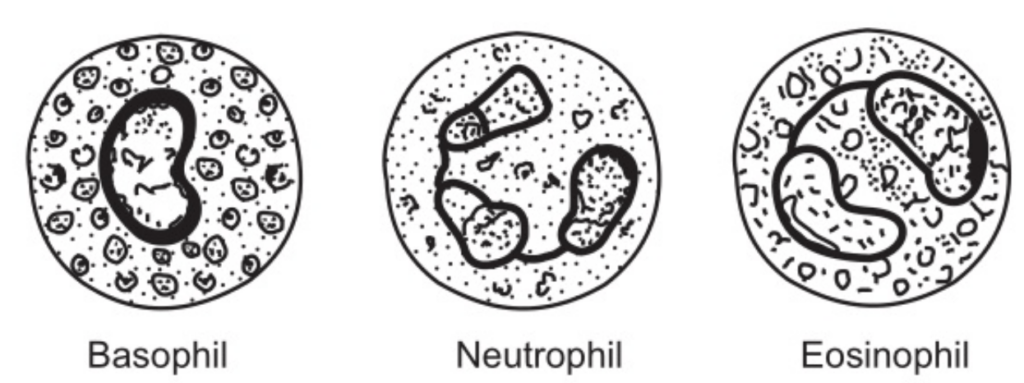
All granulocytes have multilobed nuclei. Their names represent the dyes which they take up when stained in the laboratory. Eosinophils take up the red acid dye, eosin; basophils take up alkaline methylene blue, and neutrophils are purple because they take up both dyes.
Neutrophils
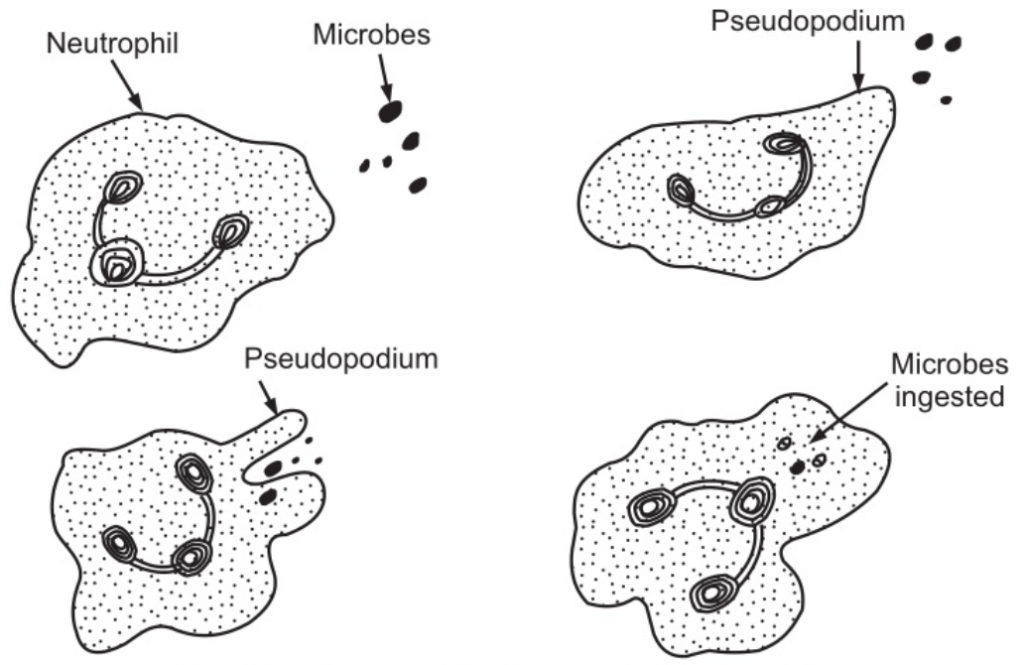
Their main function is to protect the body against any foreign material which gains entry into it, mainly microbes and to remove waste materials, e.g. cell debris. They are attracted in large numbers to any area of infection by chemical substances, released by damaged cells, called chemo toxins. Neutrophils pass through the capillary walls in the affected area by amoeboid movement. (Fig. 1.3). Subsequently, they ingest and kill the microbes by phagocytosis. (Fig.1.4)
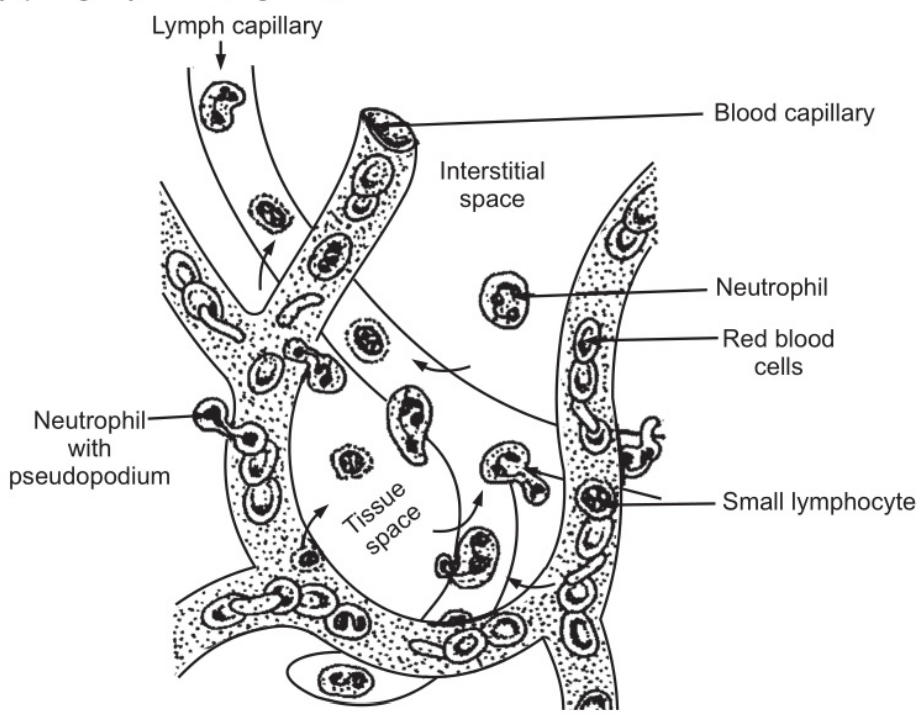
Their granules are lysosomes and contain enzymes that degrade the ingested material. The pus that may form in the affected area consists of dead tissue cells, dead and live microbes, and phagocytes killed by microbes. There is a physiological increase in circulating neutrophils following strenuous exercise and in the later stages of normal pregnancy. Numbers are also increased in the following conditions.
- Microbial infection.
- Tissue damage, e.g. myocardial infarction, burns, crushes injuries.
- Metabolic disorders, e.g. diabetic ketoacidosis, acute gout.
- Leukaemia.
- Heavy smoking.
- Use of oral contraceptives.
Neutrophils stimulate the production of interferon, which are released by virus-infected tissue cells and lymphocytes. Interferons then diffuse in tissue fluid and protect adjacent cells from invasion by viruses.
Eosinophils
Many of the eosinophils migrate out of the blood to the areas of the body exposed to the external atmosphere, i.e. connective tissue just under the skin, in the mucous membrane of the respiratory and digestive system and in the lining of the vagina and uterus. Their cytoplasmic granules are lysosomes. They are believed to protect the body against foreign materials, especially invasion by parasites. They neutralize histamine and transport plasminogen which is the precursor of plasmin. Plasmin is involved in fibrinolysis and the later stage of wound healing. The number of eosinophils is increased in allergic conditions like asthma, hay fever, food and drug sensitivities and skin conditions.
Basophils
Two major substances, i.e. histamine and heparin are present in the cytoplasmic granules of basophils. Histamine causes vasodilatation and increases the permeability of the small blood vessel wall; it also assesses in the movement of phagocytes and protective substances like antibodies, into the tissue spaces. Heparin prevents coagulation of blood. Together with mast cells basophils accumulate in the tissues in areas of local inflammation at the healing stage. Mast cells are found in the tissues, closely associated with small blood vessels and they are involved in the allergic reactions.
Agranulocytes
These are leucocytes with a large nucleus and no granules in their cytoplasm. Together they make up 25-50 per cent of all leucocytes. These are of two types:
- Monocytes
- Lymphocytes
These are large mononuclear cells originating in the red bone marrow. Some of them circulate in the blood and are actively motile and phagocytic while others migrate into the tissues where they develop into macrophages.
Both types of cell produce interleukin I which has the following functions:
- It acts on the hypothalamus, causing the rise in body temperature associated with microbial infections.
- It stimulates the production of some globulins by the liver.
- It enhances the production of activated T-lymphocytes.
Macrophages have important functions in inflammation and immunity. Macrophages function in close association with monocytes in the blood and lymphocytes which influence their activity. They are actively phagocytes, and if they encounter a large amount of foreign or waste material, they tend to multiply at the site and ‘cordon off the area, isolating the material, e.g. in the lungs when foreign material has been inhaled. Their numbers are increased in microbial infections, collagen diseases and some non-infective bowel conditions.
Lymphocytes
They have large nuclei and there are two distinct subtypes of lymphocytes: T-lymphocytes and B-lymphocytes. They circulate in the blood and are present in great numbers in the lymphatic tissue such as lymph nodes and the spleen. They develop from haemocytoblasts (stem cells) in red bone marrow, then spread into the blood to lymphoid tissue elsewhere in the body where they are activated i.e. they become immunocompetent which means that they are able to respond to antigens. Following are some of the types of antigens:
- Cells regarded by lymphocytes as abnormal, e.g. cells invaded by viruses, cancer cells, tissue transplant cells.
- Pollen from flowers and plants
- Fungi
- Bacteria
- Few large molecule drugs
The two types of lymphocytes sometimes function independently but usually in collaboration. T-lymphocytes are activated by thymosin in the thymus gland and B-lymphocytes are activated in the red bone marrow and in lymphoid tissue elsewhere in the body, possibly the walls of the intestine. Thereafter some cells of both types circulate in the blood; some settle in lymphoid tissue, mainly in lymph nodes, the spleen and the aggregated glands in the wall of the upper respiratory tract and the intestine. When activated lymphocytes encounter antigens they develop specific protective capabilities. Each type divides into two groups: effector cells that promote the destruction of their specific antigen; and memory cells that remain in lymphoid tissue and multiply, passing on their specific protective abilities to the subsequent generations of cells. The memory cells confer immunity, and subsequent encounters with the same antigen lead to a proliferation of more sensitized lymphocytes. Activated lymphocytes can respond to specific antigens by producing:
- Cell-mediated, immunity-mediated by T-lymphocytes and specialized T-cells that combat cells containing antigens.
- Humoral immunity is mediated by B-lymphocytes which involves the production of antibodies that neutralize antigens directly.
Thrombocytes or Platelets
These are very small non-nucleated disc-type cells, 02-04 µm in diameter. They are derived from the cytoplasm of megakaryocytes in the red bone marrow. They contain a variety of substances that help in the clotting of blood. The normal blood platelet count is between 200-350 × 10⁹ / 1 (200,000-350,000/mm³). The stimulus to the formation of platelets is believed to be a substance called thrombopoietin. The life span of platelets is 08-11 days, and those not used in the process of homeostasis are destroyed by macrophages, mainly in the spleen.
Homeostasis
When a blood vessel is damaged the subsequent loss of blood is stopped and healing occurs in a series of overlapping processes in which platelets plays an important role.
Vasoconstriction:
When platelets come in contact with a damaged blood vessel, their surface becomes sticky and they adhere to the damaged wall. Then they release serotonin which is also called as 5-hydroxytryptamine. It causes vasoconstriction, i.e. narrowing of blood vessels, reducing blood flow through the vessels. Other chemicals causing vasoconstriction are also released by the damaged vessel.
Platelet plug formation:
The adherent platelets clump to each other and release adenosine diphosphate (ADP) that attract more platelets to the site. Passing platelets stick to those already at the damaged vessel and they too release ADP. This is a positive feedback system whereby many platelets rapidly arrive at the site of vascular damage and quickly form a temporary seal of the platelet plug.
Blood Coagulation (Blood clotting):
This is a complex process which also involves a positive feedback system. Various factors are involved in the process of coagulation as follows:
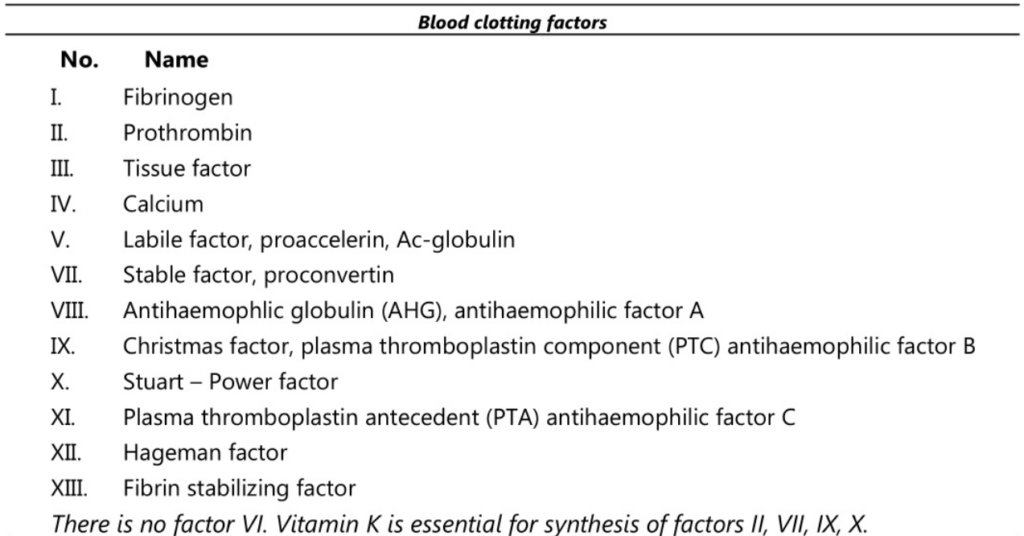
The number of the factors represents the order in which they were discovered. Blood clotting is a complex process whereby blood forms clots. Herein a damaged blood vessel is covered by a platelet and fibrin containing clot to stop bleeding and commence repair of the blood vessel. Thrombin then acts on another protein called fibrinogen and converts it to prothrombin.
Prothrombin activators can be formed by two pathways: extrinsic and intrinsic pathways. The extrinsic pathway occurs very rapidly, i.e. in seconds when there is tissue damaged outside the circulation, and it is initiated when chemicals secreted by damaged tissue enter the circulation. Relatively, the intrinsic pathway is slower and takes about 03-06 minutes. It is confined to circulation. It is triggered by damage to a blood vessel lining, i.e. endothelium and the effects of platelets adhering to it. After some time, the clot shrinks, squeezing out the serum.
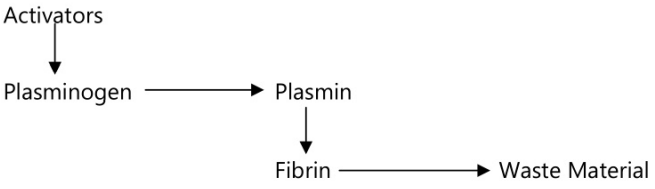
Fibrinolysis
After the formation of a clot the process of removing it and healing the damaged blood vessel begins. The breakdown of the clot or fibrinolysis is the first stage. An inactive substance called plasminogen is present in the clot and is converted to the enzyme plasmin by activators released from the damaged endothelial cells. Plasmin initiates the breakdown of fibrin to soluble products and these are treated as waste material and removed by phagocytosis. As the clot is removed, the healing process restores the integrity of the blood vessel wall.
Factors hastening coagulation of blood:
- Temperature was slightly excess than body temperature.
- Contact of blood with rough surface and injury to the blood platelets.
- Excessive slowness of blood flow.
- Snakebite.
Factors retarding coagulation of blood:
- Low temperature;
- Contact of blood with smooth surface;
- Presence of sodium or potassium citrate.
Normally, a blood clot is formed on the surface where the injury has occurred. When such a clot is formed within the circulation, it is called a thrombus. Whenever a portion of a clot gets detached and enters the circulating blood, it is called an embolus.
Make sure you also check our other amazing Article on: Axial Skeleton