Table of Contents
Paper Chromatography Principle
Paper chromatography is carried out by specially designed filter paper. The principle of separation may be here partition or adsorption. If the filter paper is impregnated with alumina or silica, the adsorption principle will be applied for separation whereas if moisture/water is present in the pores of cellulose fibre it works as stationary phase and solvent as mobile phase then the principle of separation will be partition. In the general paper, chromatography refers to the partition principle.
The procedure of the Paper Chromatography
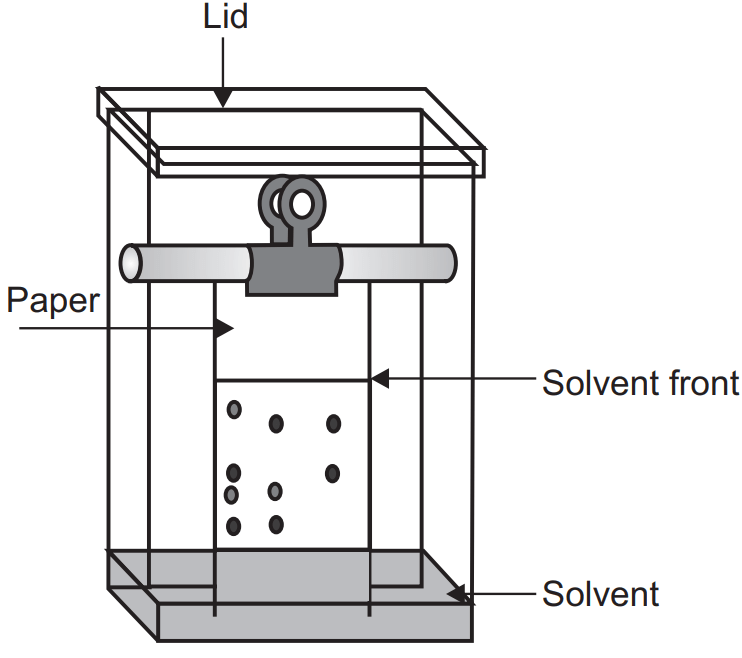
Various types of filter paper are used for paper chromatography. It may be Whatman paper of different grade, acid or base wash filter paper, paper modified with glycol, formamide, methanol, glass fibre type paper, hydrophobic paper (OH group can be acetylated) or paper can be impregnated with alumina, silica or ion exchange resin. The size of the paper should be suitable for the size of the chamber and apply the sample by using a capillary or micropipette. The sample should be dissolved in the mobile phase and applied with a low concentration with the small zone. In the mobile phase, pure solvent or a mixture of solvent or buffer solution can be used.
There may be ascending, descending, circular, two dimensional and ascending-descending type different development techniques.
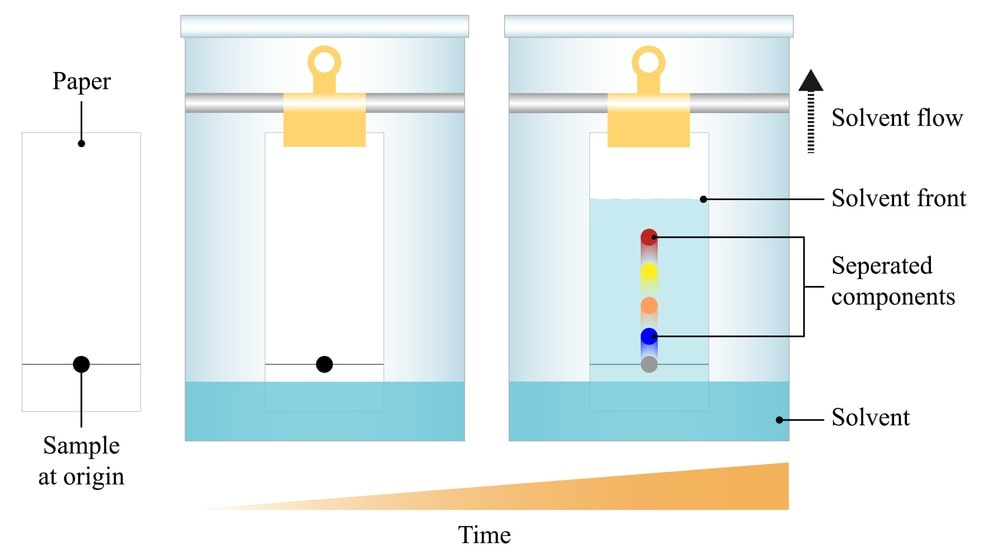
Ascending development technique is a conventional technique in which the mobile phase moves against gravity and spot the sample at the bottom portion. While in descending development the mobile phase is kept at the top and the solvent flows down the paper. Here the samples are applied at the top and the development is fast due to gravity-assisted solvent flow.
In circular or radiant development the sample is applied at the centre of the paper and the mobile phase is flown through a wick at the centre and spread uniformly in all directions. In two-dimensional development techniques, the samples are applied in one corner and develop the paper on one axis then dry the paper. After drying turn the paper on a ninety-degree angle and develop the paper on another axis. This technique is used for the more complex sample. By ascending descending development the length of separation can be increased. First here ascending takes place later descending development follow.
After the development of the chromatogram, the isolated compound can be visualized by detecting agents.
Detecting agents can be two types:
(a) Destructive type
(b) Non-destructive type
In destructive type, the sample cannot be recovered or it will be destroyed due to the chemical reaction of spraying reagent with the sample. While in the non-destructive method sample can be recovered. In the non-destructive method, the sample can be detected by the UV chamber method, densitometric method or iodine chamber method.
Paper chromatography can be used for both qualitative and quantitative purposes. For qualitative purposes, the Rf value can be determined.

For the quantitative purpose, the density of the spot can be measured or the spot can be eluted with the solvent and analysed by conventional techniques like spectrophotometric method or electrochemical methods.
Paper Chromatography Applications
- To investigate the fermentation and ripening processes.
- To ensure that medications are pure.
- To examine cosmetics.
- Adulterants must be detected.
- To identify pollutants in beverages and meals.
- In biochemical laboratories, to examine reaction mixtures.
Make sure you also check our other amazing Article on : Column Chromatography