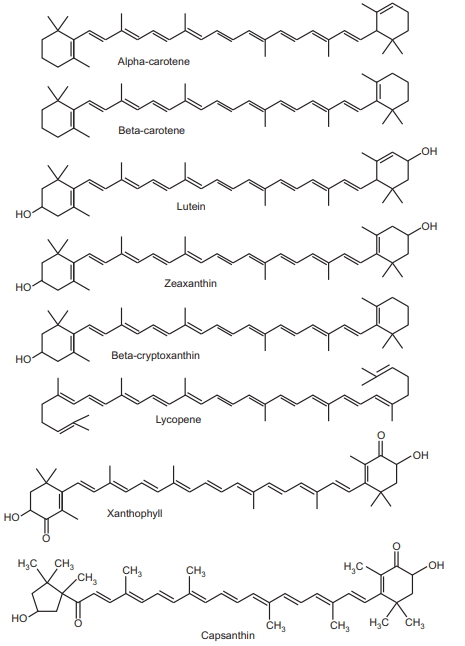
Carotenoids include many tetraterpenoid compounds which consist chain of eight isoprene units. The color and oxidizing tendency of carotenoids mainly depend on the presence of characteristic chromophores which contain at least ten conjugated double bonds. The yellow or orange color mainly depends on the presence of a particular chromophore. ‘Carotenes’ are the hydroxylated derivatives of Xanthophylls. Carotenoid Structure are acyclic like lycopene or comprise one or two pentacyclic or hexacyclic rings at one end or other ends i.e. β, ψ-carotene, or both ends like β, β-carotene. These pigments widely occur and accumulate in the chloroplast of photosynthetic tissues. β-carotene, neoxanthin, and lutein are a few examples which accumulate in the leaf part of the plant whereas some chloroplastic carotenoids or their derivatives are accumulated in fruits (e.g. capsanthin found in capsicum, lycopene found in tomato, apocarotenoids found in citrus fruits), flower petals (e.g. marigold, French marigold), roots (carotene found in carrot roots) and seeds (e.g. zeaxanthin found in corn).
Carotenoid involved in photosynthesis absorbs and transmits photon radiation (450-500 nm), provides protection against harmful radiations (prevents photo-oxidation by reacting with singlet oxygen), act as antioxidants (reacts with peroxy free radicals). Retinol or Vitamin A is produced by the degradation of β-carotenes in human intestinal mucosa and fulfills the requirement of the body. Carotenoids also prevent from degenerative disorders. They are also used in porphyria photosensitization (because interferes in photo-oxidation processes), dermatitis of phototoxic origin, urticaria, lupus erythematosus, etc.
In pharmaceutical industries, it is used as a natural, efficacious, non-toxic coloring agent (e.g. annatto extract, lycopene, carotene, xanthophylls, etc). Pure carotenoid occurs in two forms-
- As in the form of a microcrystalline suspension in vegetable oil
- In the form of a powder dispersed in water.
The application of carotenoids in food industries, other than pharmaceutical industries, are in dairy products, pastries, soup and candies preparations, liquors, and beverages preparations, etc. In animal feed industries the pigmentation of poultry meat and the color of eggs can be enhanced by the use of carotenoids. The natural carotenoids occur in extract form like paprika extract. It has no acceptable daily intake limit but others have a limit (5 mg/Kg).
Table of Contents
Carotenoid Structure of Lycopene, Carotene, Xanthophylls, Capsanthin, Lutein
Classification of Carotenoid
Chemically, carotenoids are fat-soluble plant pigments which provide color in nature. These are polyisoprenic compounds which consist of isoprene units nearly eight carbon atoms and forty carbon atoms.
There are nearly 600 compounds which exists in the carotenoids group which can be classified as:
- Carotenes or carotenoids: In these pigments only carbon and hydrogen atoms are present.
- Xanthophylls: These pigments are oxygenated hydrocarbon compounds which contain at least one oxygen atom, hydroxyl, keto, epoxy, methoxy, or carboxyl groups.
- Apocarotenoids: These pigments are typically formed by cleavage of carotenoids.
Properties of Carotenoids
- Carotenoids are found in the chloroplast in green plants as part of photosynthesis but they are more visible and more colored in roots, fruits, and flowers.
- Carotenoids are yellow, red, or orange in color which occurs in both plants and animals.
- They are synthesized in plants but in animals, they originate from foods of plant origin.
- They occur in a free state as well as in combination with holoprotein and carbohydrates (carotenoproteides, carotene glycosides).
- Due to hydrocarbon structure, these compounds are hydrophobic in nature and solubilizes only in organic solvents, oils, and fats.
- They are characterized by the presence of a conjugated double bond structure.
- They are unsaturated in nature and cause oxidation and autoxidation reactions in the open air.
- Carotenoid pigments play an important role in plant photosynthesis and in the protection of self-photo destruction of the chlorophyll molecule and other active substances like cytochromes, peroxidases, catalases, vitamin B 12, vitamin E, and vitamin K.
Naturally occurring Carotenoids
- Hydrocarbons: Hexahydrolycopene, Lycopersene, Phytofluene, Torulene, etc.
- Alcohols: Alloxanthin, Crustaxanthin, Cryptomonaxanthin, cynthiaxanthin, Lutein, etc.
- Glycosides: Oscillaxanthin and Phleixanthophyll.
- Ethers: Rhodovibrin and Spheroidene.
- Epoxides: Citroxanthin, Diadinoxanthin, Luteoxanthin, Zeaxanthin, etc.
- Aldehydes: Rhodopinal. Torularhodin methyl ester.
- Acids and acid esters: Torularhodin and torularhodin methyl ester.
- Ketones: Phoenicoxanthin, Astacene, Astaxanthin, Capsanthin, Flexixanthin, etc.
- Alcohol esters: Astacein, Flucoxanthin, Isoflucoxanthin, Physalien, Siphonein, etc.
- Apocarotenoids: Bixin, Paracentrone, Lycopenoate, Crocetin, etc.
Extraction of Carotenoids
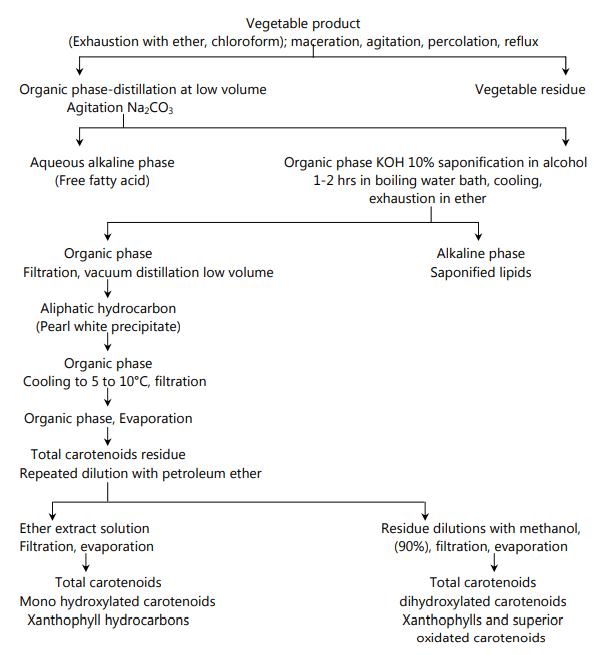
Methods of Isolation
Isolation methods depend on the physical and chemical properties of the bio substances to be separated. Commonly used methods of separation are:
- Distillation: It is a process of separation of constituents from a liquids mixture by selective evaporation and condensation.
- Crystallization: It is the process of formation of solid crystals precipitating from a solution that melts or is deposited directly from a gas.
- Electrophoresis: It is a method of separation and analysis of macromolecules and their fragments, based on their size and charge/separates organic molecules based on their different interaction with a gel under an electric potential.
- Column chromatography and thin-layer chromatography: This is a physical method of isolation which distributes components to separate between two phases, one stationary phase and other is mobile phase moving in a definite direction; subtle differences in a compounds partition coefficient which results in differential retention on the stationary phase and changed the separation.
Make sure you also check our other amazing Article on : Extraction of Volatile Oils
