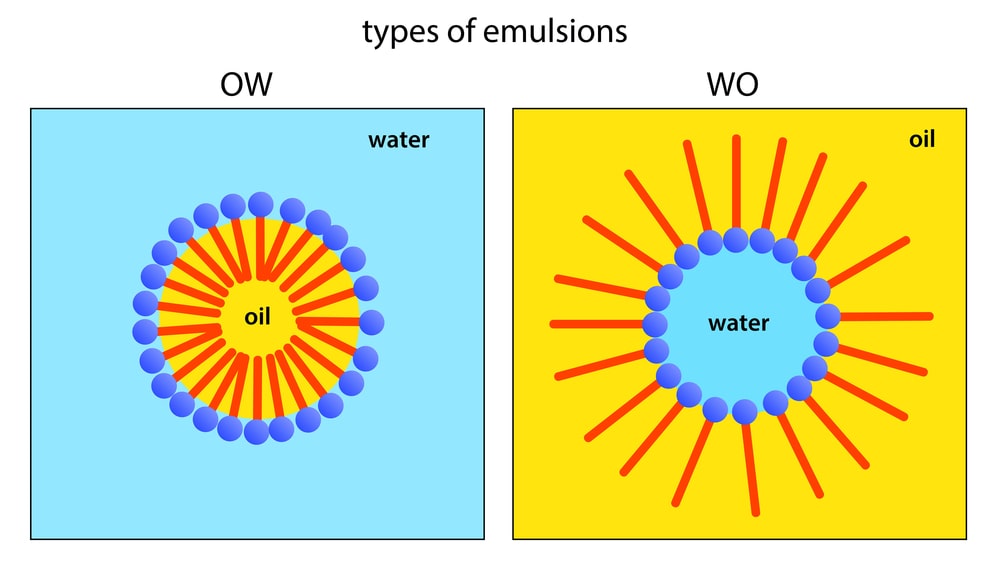
An emulsion is a liquid preparation containing two immiscible liquids, one of which is dispersed as globules (dispersed phase = internal phase) in the other liquid (continuous phase = external phase).
- Droplets ranging in diameter (0.1-100 um).
- The emulsion is thermodynamically unstable and is stabilized by the presence of emulsifying agent (emulgent or emulsifier).
- The emulsion is no more official in L.P. Emulsion protect drug which is susceptible to hydrolysis and oxidation.
- It also provides prolonged action of the medication. In the form of an o/w emulsion, ephedrine has a more prolonged effect when applied to the nasal mucosa, than when used in an oily solution.
Table of Contents
Types of Emulsion
A primary emulsion containing one internal phase, for example,
- oil-in-water emulsion (o/w)
- water-in-oil emulsion (w/o).
The secondary emulsion also called multiple emulsion contains two internal phases, for instance,
- o/w/o
- w/o/w.
Test for Emulsion
Click on the link given below and read the full article: Identification Test For Emulsion
Theories of Emulsification
In the case of two immiscible liquids, the cohesive force between the molecules of each separate liquid exceeds the adhesive force between two liquids. This is manifested as interfacial energy or tension at the boundary between the liquids.
Therefore, to prevent the coalescence and separation, emulsifying agents have been used.
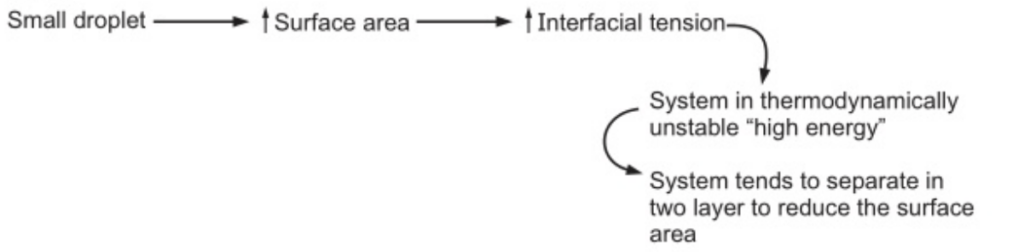
Surfactant: Adsorbed at oil/water interface to form monomolecular film to reduce the interfacial tension. e.g., Tween and Spans.
Hydrophilic colloids: Forming a multimolecular film around the dispersed droplet. e.g., Acacia.
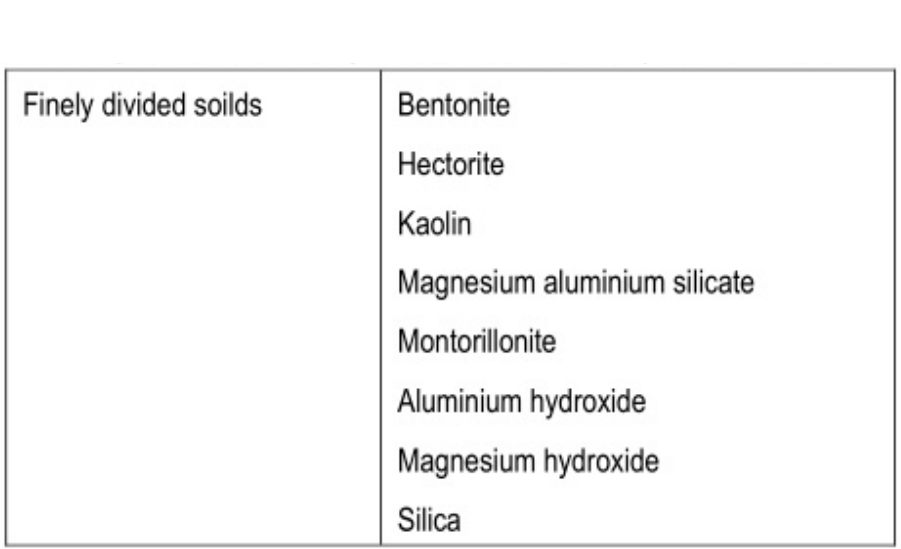
Finely divided solids: They are adsorbed at the interface between two immiscible liquid phases to form the particulate film. e.g., Bentonite and veegum.
Monomolecular adsorption:
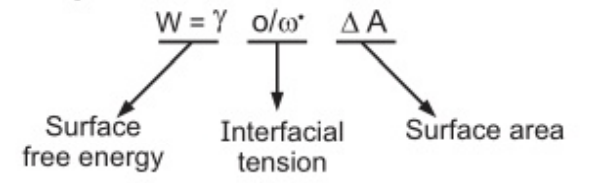
The surface-active agent (SAA) is a molecule which has two parts, one is hydrophilic and the other is hydrophobic. Upon the addition of SAA, they tend to form monolayer film at the oil/water interface.
The functions of surface-active agents to provide stability to dispersed droplets are as follows:
- Reduction of the interfacial tension.
- Form a coherent monolayer to prevent the coalescence of two droplets when they approach each other.
- Provide surface charge which causes repulsion between adjust particles.
Bancroft rule
As per the Bancroft rule, the emulsifying agent is used in an emulsion should be favorable to the external phase of the emulsion.
So even though there may be a formula that is 60% oil and 40% water, if the emulsifier chosen is more soluble in water, it will create an oil-in-water system.
The Hydrophilic-Lipophilic Balance (HLB) of a surfactant can be used in order to determine whether it is a good choice for the desired emulsion or not.
In Oil in Water emulsions, use emulsifying agents that are more soluble in water than in oil (High HLB surfactants).
In Water in Oil emulsions, use emulsifying agents that are more soluble in oil than in water (Low HLB surfactants).
Multimolecular adsorption
Hydrophilic colloids form multimolecular adsorption at the oil/water interface. They have a low effect on the surface tension.
- Their main function as emulsion stabilizers is by making the coherent multi-molecular film. This film is strong and resists the coalescence. They have, also, an auxiliary effect by increasing the viscosity of the dispersion medium.
Solid particle adsorption
Finely divided solid particles are adsorbed at the surface of the emulsion droplet to stabilize them. Those particles are wetted by both oil and water (but not dissolved) and the concentration of these particles forms a particulate film that prevents the coalescence.
Emulsion Stability
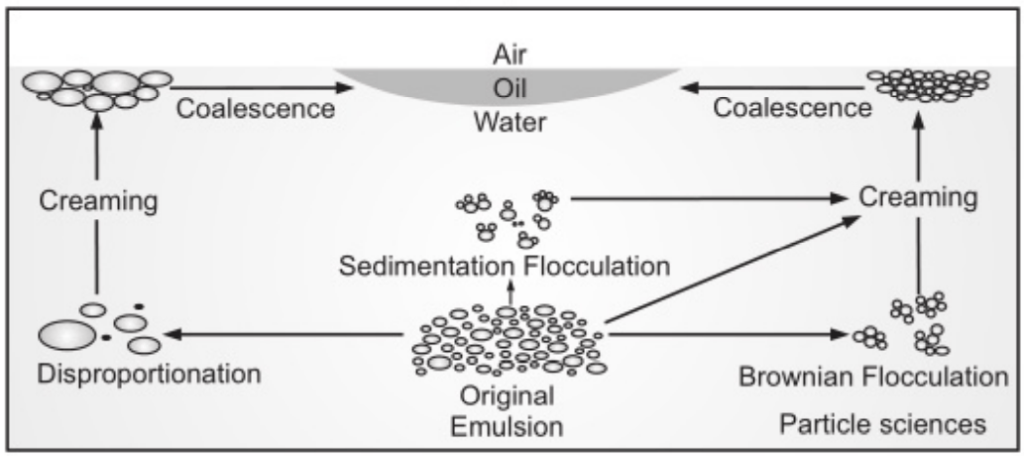
The process by which an emulsion completely breaks is generally considered to be governed by four different droplet loss mechanisms, i.e.
- Brownian flocculation,
- Creaming,
- Sedimentation flocculation and disproportionation.
Creaming – upward and downward
- Creaming derives its name from the most commonly known example emulsification process.
- The separation of milk into its cream and skim milk components. Creaming is not an actual breaking but a separation of the emulsion into two emulsions, one of which (the cream) is richer in the disperse phase than the other. Creaming is the principal process by which the disperse phase separates from an emulsion and is typically the precursor to coalescence
- The creaming rate (or settling rate for dispersing phases denser than the continuous phase) can be estimated from the Stoke’s equation:
v = 2r² (p-po) g/9n
where v is the creaming (settling) rate, r is the droplet radius, p is the density of the droplet, po is the density of the dispersion medium, n is the viscosity of the dispersion medium (continuous phase) and g is the local acceleration due to gravity.
Flocculation
- The aggregation of droplets to gives 3-D clusters without coalescence occurring. Importantly, all droplets maintain their own integrity and remain as totally separate entities. It results when there is a weak, net attraction between droplets and arises through the various machines.
- Flocculation may be subdivided for convenience into two general categories: that resulting from sedimentation aggregation and that from Brownian motion aggregation of the droplets.
Disproportionation or Ostwald ripening
- It is dependent on the diffusion of dispersed phase molecules from smaller to larger droplets through the continuous phase.
- The pressure of dispersed material is greater for smaller droplets than larger droplets as per the Laplace equation.
Coalescence
- A few globules tend to fuse with each other and form bigger globules.
- In this process, emulsifier film around the globules is destroyed to some extent.
Breaking
- Complete separation of phases, irreversible process. Phase Inversion
In phase inversion o/w type emulsion changes into w/o type and vice versa. It is a physical instability.
- It may be brought about by the addition of an electrolyte, by changing the phase volume ratio or by temperature changes, by changing the chemical nature of the emulsifier Phase inversion can be minimized by using the proper emulsifying agent in adequate concentration, and by storing the emulsion in a cool place.
Preparation of Emulsions
The preparation of emulsions depends on the scale at which it is produced.
On a small scale, mortar and pestle can be used but its efficiency is limited. To overcome this drawback small electric mixers can be used although care must be exercised to avoid excessive entrapment of air.
For large-scale production mechanical stirrers are used to provide controlled agitation and shearing stress to produce stable emulsions.
The methods commonly used to prepare emulsions can be divided into two categories:
Trituration Method
This method consists of the dry dum method and the wet gum method.
Dry Gum Method
In this method, the oil is first triturated with gum with a little amount of water to form the primary emulsion. The trituration is continued till a characteristic ‘clicking’ sound is heard and a thick white cream is formed. Once the primary emulsion is formed, the remaining quantity of water is slowly added to form the final emulsion.
Wet Gum Method
As the name implies, in this method first gum and water are triturated together to form a mucilage. The required quantity of oil is then added gradually in small proportions with thorough trituration to form the primary emulsion.
Once the primary emulsion has been formed remaining quantity of water is added to make the final emulsion.
Bottle Method
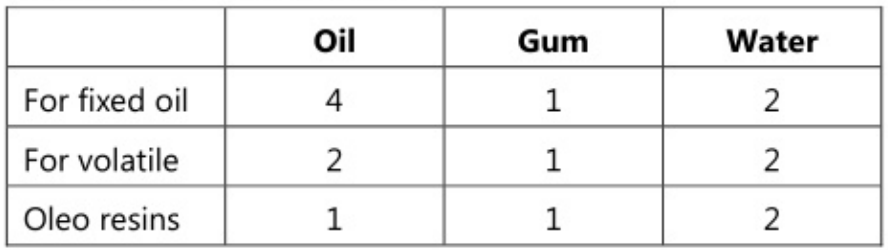
This method is employed for preparing emulsions containing volatile and other non-viscous oils. Both dry gum and w gum methods can be employed for the preparation. As volatile oils have a low viscosity as compared to fixed oils, they require a comparatively large quantity of gum for emulsification.
In this method, oil or water is first shaken thoroughly and vigorously with the calculated amount of gum. Once this has been emulsified completely, the second liquid (either oil or water) is then added all at once and the bottle is again shaken vigorously to form the primary emulsion. More of water is added in small portions with constant agitation after each addition to produce the final volume.
Formulae for Primary Emulsion
Dry gum method or continental method
Evaluation of Emulsion
Stability testing of emulsions involves determining stability at long-term storage conditions, accelerated storage conditions, freezing and thawing conditions. Stress conditions are applied in order to speed up the stability testing.
- The stress conditions used for speeding up instability of emulsions include:
- Centrifugal force, Agitational force, Aging, and temperature.
2. Physical parameters are evaluated to assess the effect of any of the above stress conditions:
- Phase separation
- Viscosity
- Electrophoretic properties
- Particle size and particle count
1. Determination of particle size and particle count: Determination of changes in the average particle size or the size distribution of droplets is an important parameter used for the evaluation of emulsions. It is performed by optical microscopy, sedimentation by using Andreasen apparatus and Coulter counter apparatus.
2. Determination of viscosity: Determination of viscosity is done to assess the changes that might take place during aging. Emulsions exhibit the non-newtonian type of flow characteristics.
The viscometers which should be used include cone and plate viscometers.
3. Determination of phase separation: This is another parameter used for assessing the stability of the formulation.
Phase separation may be observed visually or by measuring the volume of the separated phases.
4. Determination of electrophoretic properties: Determination electrophoretic properties like zeta potential is useful for assessing flocculation since electrical charges on particles influence the rate of flocculation.
Types of Surfactants
- Anionic
- Cationic
- Non-ionic
Anionic: Monovalent, polyvalent, and inorganic soaps sulfates and sulphonates, alkali soaps include sodium, potassium, and ammonium salts are lauric, myristric, palmitic, stearic and oleic acid is water-soluble which forms o/w emulsion.
Metallic soaps like calcium or magnesium salts are fatty acids that are water-insoluble and tend to form w/o emulsion. Organic soaps form o/w emulsion. Sulfated alcohols such as sodium lauryl sulfate form o/w emulsion.
Sodium dioctyl sulphosuccinate is frequently used sulphonates di-(2-ethyl hexyl) sodium sulphosuccinate called Aerosol OT.
Cationic emulgent: They are commonly used in lotion and cream due to their remarkable bactericidal property. For example domiphen bromide, cetyl pyridinium bromide, benzalkonium chloride, cetyl trimethyl ammonium bromide.
Non-ionic emulgents: For example, Glyceryl ester, fatty acid esters of sorbitol, and their poly oxyethylene derivative, polyoxyethene glycol esters, and Sorbitan fatty acid esters e.g. Sorbitan mono palmitate (span-40) are non-ionic and oil soluble promoting w/o emulsion.
Polyoxyethylene sorbitan monopalmitate (tween 40) are hydrophilic, water soluble derivative that promotes o/w emulsion.
- Amphoteric: N-dodecyl, N, N-dimethyl betaine, lecithin.
PEG esters such as polyoxy ethylene glycol 400 monostearate are widely used to prepare emulsified lotion and creams.
- Natural emulgents: Most commonly used is acacia. Others are Gelatin which is amphoteric in nature, Lecithin (phospholipid), and Cholesterol.
- Acacia and gelatin: Form interfacial monolayer.
- Lecithin and cholesterol: Folg interfacial mono molecular layer. Lecithin is a phospholipids and forms o/w emulsion, Darken on storage.
- Cholesterol form: w/o emulsion.
- Finely dispersed solids: Colloidal clays such as bentonite, veegum, oxide, silica gel, aluminum hydroxide, Magnesium oxide. Magnesium hydroxides are the most commonly used finely dispersed solids which act by forming a particular particulate film around dispersed globules.
- Bentonite produces both o/w and w/o types of emulsion depending on the order of mixing.
- Veegum is also as an emulgent for o/w emulsion but it is chiefly used as a stabilizer in cosmetic creams and lotion.
- Auxiliary emulgents are incapable of forming a stable emulsion. Have thickening property. Thus, the consistency of an o/w emulsion was prepared by using acacia. It can be increased by tragacanth or agar which act as an auxiliary emulsion.
List of antioxidants (0.001-0.1%)
- Gallic acid
- Propyl Gallate
- Ascorbic acid
- Sulfites
- alpha-Tocopherol
- Butylated hydroxyl toluene
- Butylated hydroxy anisole
- Ascorbyl palmitate
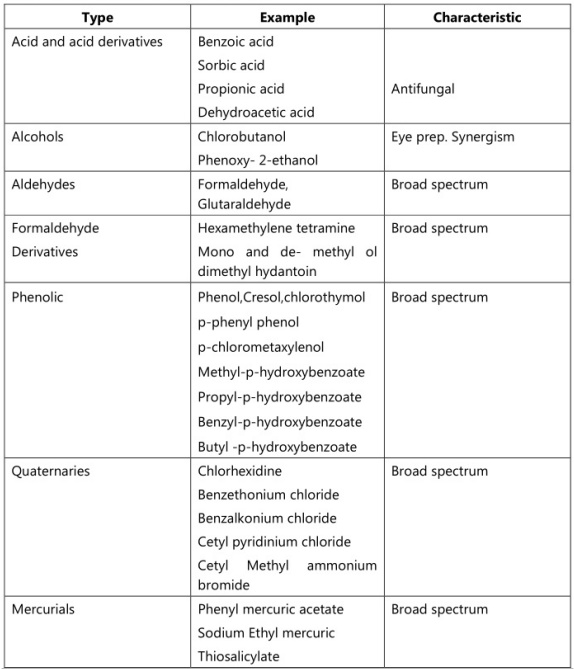
The preservative used in Emulsion
Make sure you also check our other amazing Article on : Coating Of Tablets