The microbiological assay is a technique for assessing a compound’s potency or concentration by observing its effect on a microbe. In both the British and American Pharmacopoeias, it is a legal quality control criterion for the assay of a number of antibiotics. Microbiological assays are used to standardize a variety of medicinal drugs that either inhibit or are required for the growth of microorganisms (antibiotics, vitamins, and amino acids).
The Microbiological Assay and Methods of an antibiotic are based on a comparison of the inhibition of microorganism growth caused by measured concentrations of the antibiotic under investigation with that caused by a known concentration of a standard preparation of the antibiotic with known activity.
Microbiological assay of vitamins is a type of biological assay performed with the help of microorganisms.
Applications
- This method determines the potency.
- The method controls antimicrobial chemotherapy.
- Good in-vitro and in-vivo correlations are provided by this method.
- This method is accurate, inexpensive, and convenient.
- This method is easy to interpret results.
Two methods are used for the Microbiological Assay and Methods namely cylinder plate or cup plate method and tube assay method or turbidity method.
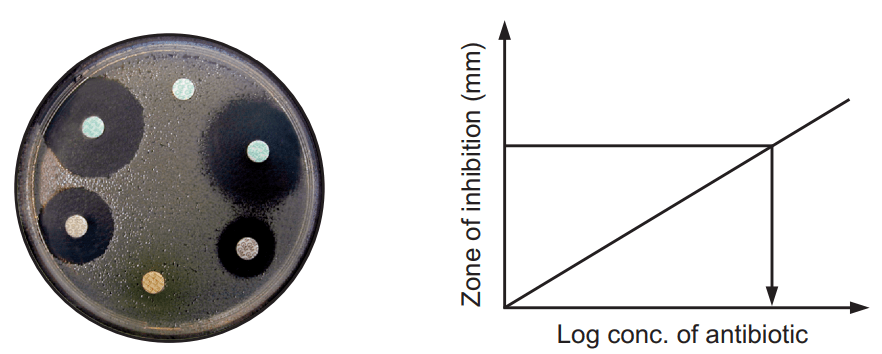
Method A: Cylinder Plate Method
Principle
This method depends on the diffusion of an antibiotic from a vertical cavity through the solidified agar layer in the Petri plate. The growth of test microorganisms is inhibited entirely in a zone around the cylinder containing antibiotic solution.
Procedure:
- The nutrient agar is melted, cooled, and poured into the petri dish.
- 0.2 ml of known concentration of inoculum is spread on the surface of solidified agar by spread plate technique.
- Holes or cavities are made by a sterile borer.
- 0.2, 0.4, 0.6, 0.8, and 1 ml of antibiotic is poured into the cup of the agar plate and then incubated in 37°C for 24 hours.
- A zone of inhibition is observed for the antibiotic that has antimicrobial activity.
- The zone of inhibition is measured by scale from the backside of the plate from the center of the cavity or hole.
One Level Assay:

Where,
L = The calculated zone diameter for the lowest concentration of the standard curve response line.
H = The calculated zone diameter for the highest concentration of the standard curve response line.
c = Average zone diameter of 36 readings of the reference point standard solution.
a, b, d, and e = Corrected average values for the other standard solutions, lowest to highest concentrations, respectively.
Two Level Factorial Assay
Parallel dilutions containing two levels of both the standard (S1, and S2) and unknown (U1 and U2) are prepared. Cavities of each of plate are poured with diluted test samples along with standard. Finally, they are kept in the incubator, and the zone of inhibition is measured. The potency is calculated by the following formula
% potency = Antilog (2.0 + a log I)
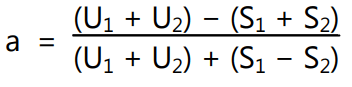
Where,
U1 and U2 = The sums of the zone diameters with solutions of the unknown of high and low levels.
S1 and S2 = The sums of the zone diameters with solutions of the standard of high and low levels.
I = Ratio of dilutions.
If the potency of the sample is less than 60 percent or more than 150 percent of the standard, the assay is invalid and should be repeated using higher or lower dilutions of the same solution. The potency of the sample may be calculated from the expression.
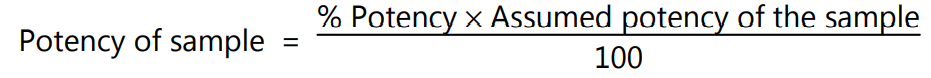
Method-B: Tube Assay Method or Turbidity Method
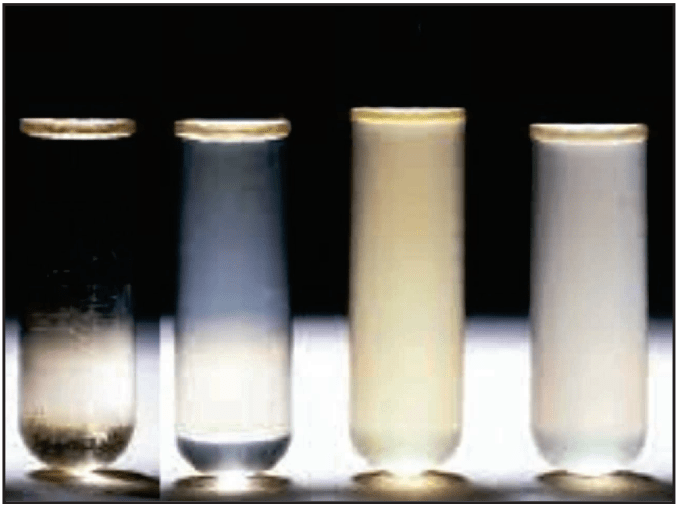
The method is based on the correlation between turbidity and changes in the microbial cell number. The turbidity is measured in a suitable liquid medium inoculated with microorganisms and finally, standard optical density or turbidimetric curves are used to estimate the number of microbial cells (Fig. 1.2).
Principle
This method depends on the inhibition of growth of microbial culture in a uniform solution of the antibiotic in the fluid medium that is favorable to its rapid growth in the absence of antibiotics.
Procedure
- Five different concentrations of the standard solutions are prepared from the stock solution of standard as per step-wise increased ratio 4: 5.
- The concentration of media is selected and unknown substance solution is diluted to that concentration.
- 1 ml of each concentration of the standard solution as well as test sample solution in each of the tubes is placed in duplicate.
- 9 ml of nutrient medium is added to each of the tubes.
- Side by side three control samples are prepared. One contained the inoculated culture medium, another is blank (0.5 ml of dilute formaldehyde solution) and the third one contains an uninoculated culture medium.
- All the tubes are placed in the incubator for 3-4 hours at 37°C and after incubation 0.5 ml of dilute formaldehyde is added in each tube.
- The growth of the test microorganism is measured by determining the absorbance at about 530 nm of each of the solutions in the tubes against the blank.
Calculation

L = The calculated absorbance for the lowest concentration of the standard response line.
H = The calculated absorbance for the highest concentration of the standard response line.
a, b, c, d, and e = Average absorbance values for the each concentration of standard response line, lowest to highest concentrations, respectively.
Advantage:
The shorter incubation period (3-4 hours) for the growth of the test organism.
Disadvantage:
The presence of solvent residues inhibitory substances affects more in this assay. Further, this method is not applicable for the turbid or cloudy preparation.
Make sure you also check our other amazing Article on : Sources of Contamination & its prevention in an Aseptic Area