Friedel Crafts Acylation: The acylation reaction is the replacement of the hydrogen atom of certain functional groups ie, the hydroxyl, amino and other groups, by the residue of carboxylic acid:
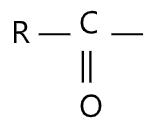
Esters are obtained from the acylation of alcohol and mono and di-substituted amides are obtained in amine acylation. Preparation of aromatic ketones by the interaction of aromatic hydrocarbons with acid chlorides or anhydrides in the presence of aluminium chloride is called as Frieder-Craft’s acylation. This is an electrophilic substitution reaction in which the RCO-, and ArCO- moieties, referred to as acyl groups are introduced onto the rings. When an aromatic compound is treated with acid chloride and aluminium trichloride, an aromatic ketone is formed.
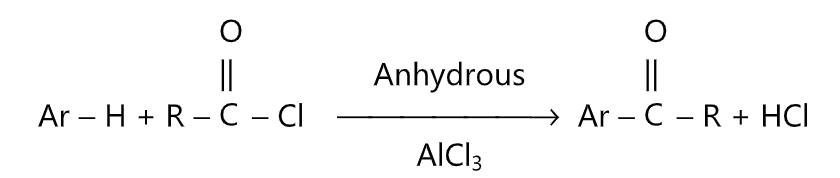
Aluminium trichloride acts as a Lewis acid and removes chlorine from the acid chloride to produce the electrophile. The effective electrophile may be the acylium ion or a polarised complex. The reaction conditions (ie, polarity of solvent and size of R) govern the nature of the electrophile.
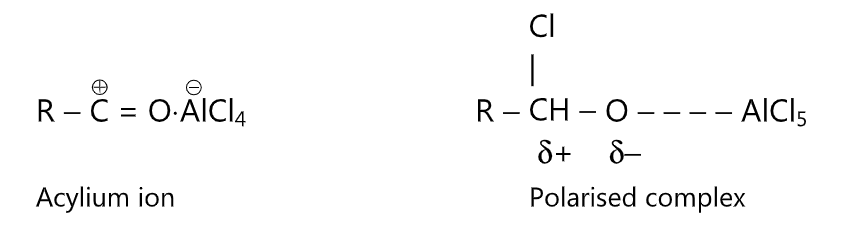
The reaction is carried out under anhydrous conditions. More than 1 mole equivalent of AlCl3, must be used for each mole of acid chloride.
The general mechanism of reaction follows as below:
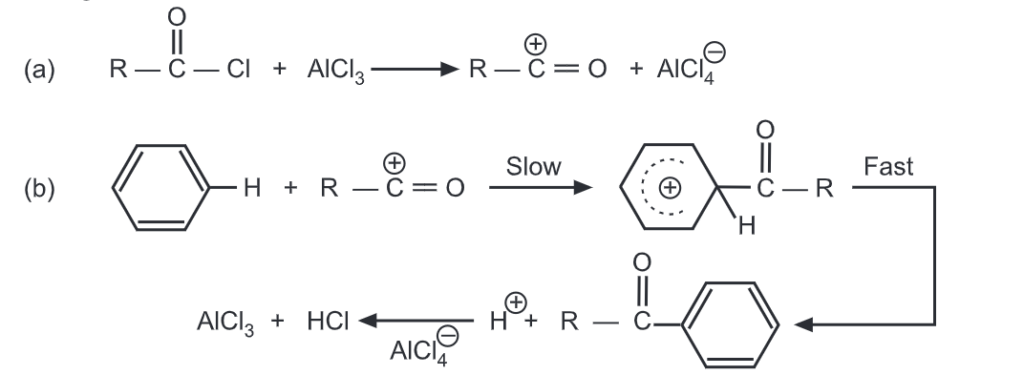
Since the reaction is exothermic, acid chloride or anhydride should be added dropwise to the mixture. After the acylating agent is added, the reaction mixture is heated in a water bath for 1-3 hours and then poured out on the ice and treated with hydrochloric acid. The ketone isolated is extracted by a solvent, the water layer is separated, and then ketone is dried and distilled.
The aluminium in AlCl3 coordinates with the carbonyl oxygen of the ketone. The ketone is liberated by the addition of water which breaks up the complex.
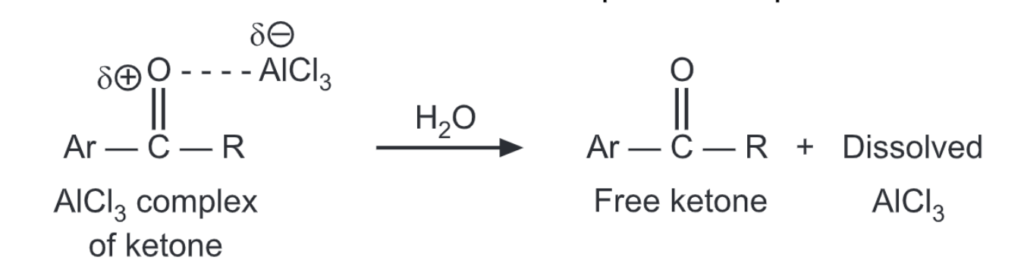
Unlike Friedel-Craft’s alkylation, the acylation reaction does not undergo rearrangement. It can not be carried out on a deactivated (ie, having strongly deactivating meta directors) aromatic ring. For example, benzoyl chloride can not acylate m-dinitrobenzene because of the presence of deactivating nitro groups. For the same reason, the introduction of a second acyl group onto the ring is impossible. However, the acid chloride containing one or more strongly deactivating groups may be used.
Besides acid chloride, acid anhydride may also be used to carry out acylation.
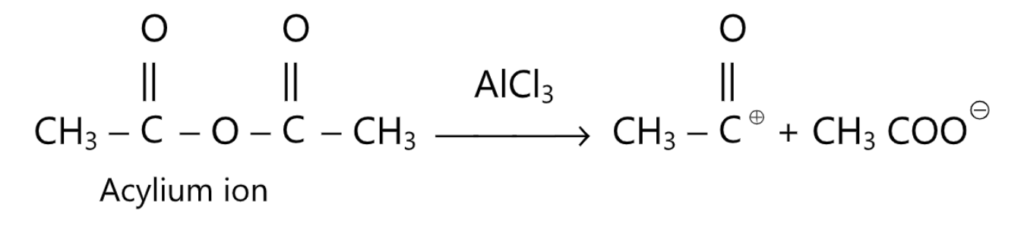
Unlike alkylation by Friedel-Craft’s method, for which the presence of a small amount of aluminium chloride is enough, acylation occurs satisfactorily only when the amount of AlCl3, used is far greater than that used for alkylation. In acylation by acid anhydrides, an even larger amount of AlCl3 must be used (i.e., more than 2 moles of AlCl3, per mole of acid anhydride) because the ketone and acetic acid, formed as a result of the reaction, bind the same amount of the catalyst.
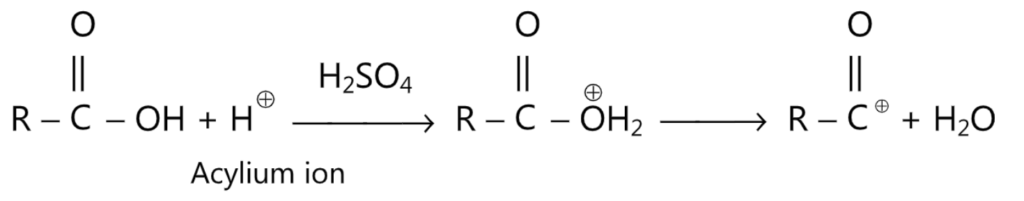
The acylium ion may also be generated from carboxylic acids by the action of conc. H2SO4 or HF through protonation reaction.
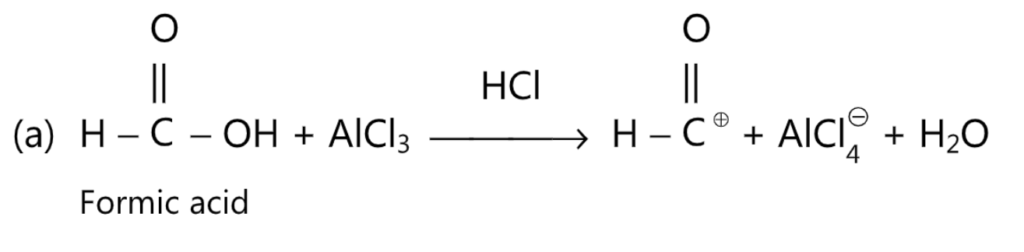
While carrying out formylation, the Gattermankoch reaction is suggested in which HCOOH, HCI and AlCl3, are used.
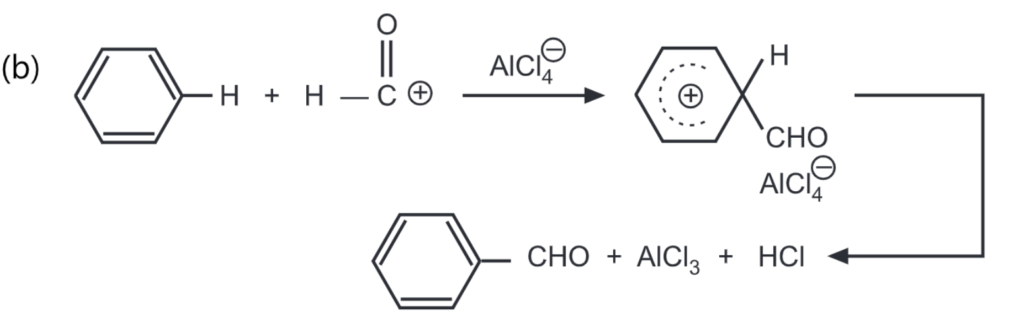
Since many organic solvents react with AlCl3, the selection of a proper solvent for Friedel Craft’s reaction is problematic. Usually, carbon disulphide, nitrobenzene, petroleum ether or excess aromatic hydrocarbon is used.
Make sure you also check our other amazing Article on : Sulfonation of Benzene