Geometrical Isomers are also called cis-trans isomerism. Maleic acid (m.p. 130°C) and fumaric acid (m.p. 287°C) have the same molecular formula but differ in the arrangement of functional groups around the double bond. They have different physical and, to some extent, chemical properties. This type of isomerism is known as geometrical isomerism.
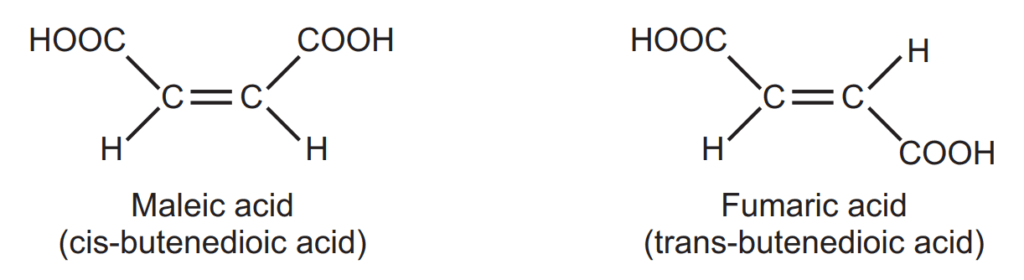
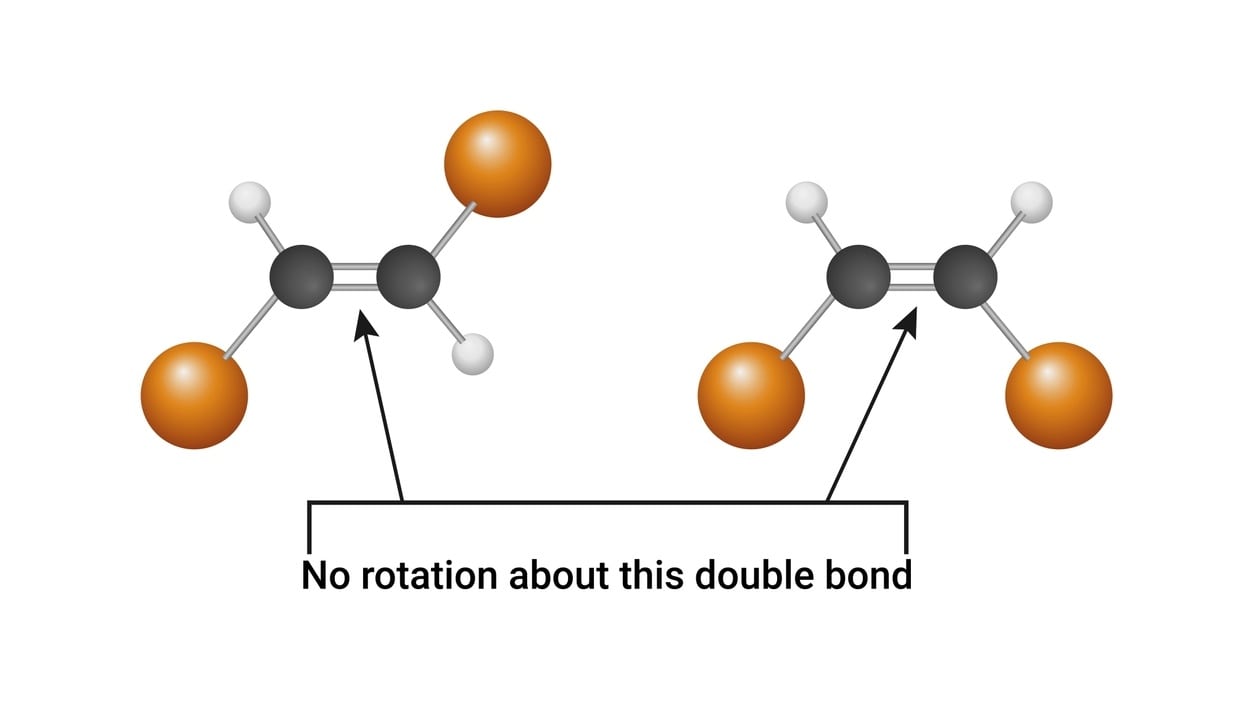
The presence of a carbon-carbon double bond restricts the freedom of rotation about the double bond. The designation cis (Latin word: same side), is used to denote the presence of like atoms or groups on the same side and trans (Latin word, across) is used when they are on opposite sides. Isomerism seen in non-cyclic, open-chain compounds due to the presence of a double bond, is called π diastereoisomerism while when it occurs in a cyclic skeleton lacking a double bond, it is termed σ diastereoisomerism.
Make sure you also check our other amazing Article on : Reactions of anthracene