The use of liquid pharmaceuticals has been justified on the basis of ease of administration and rapid and efficient absorption of the drug. Dosage forms meant either for internal, external or parenteral use may be sub-classified into monophasic or biphasic liquid dosage forms. The monophasic liquid dosage forms consist of either true or colloidal solutions or solubilized systems. All these consist of only a single phase and may have either aqueous or non-aqueous solvents as the base. Biphasic dosage forms are represented by emulsions and suspensions and consist of two immiscible phases, the continuous phase, and the dispersed phase. The continuous phase in both is a liquid, the dispersed phase in emulsions is also a liquid while in the case of suspensions, the dispersed phase consists of a finely divided solid. The classification of the liquid dosage form is given in Fig 1.1. and the comparison of characteristics of various liquid dosage forms is shown in Table 1.1.
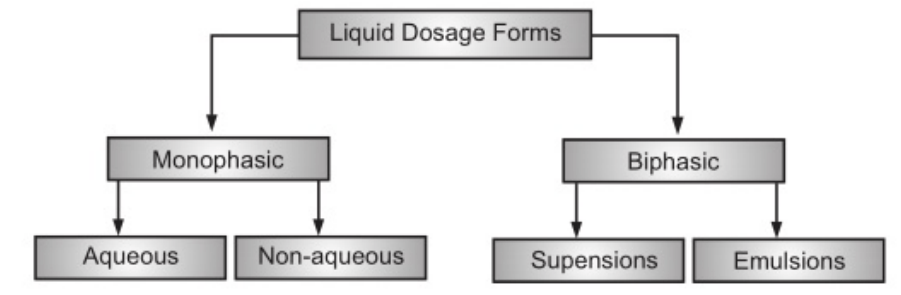
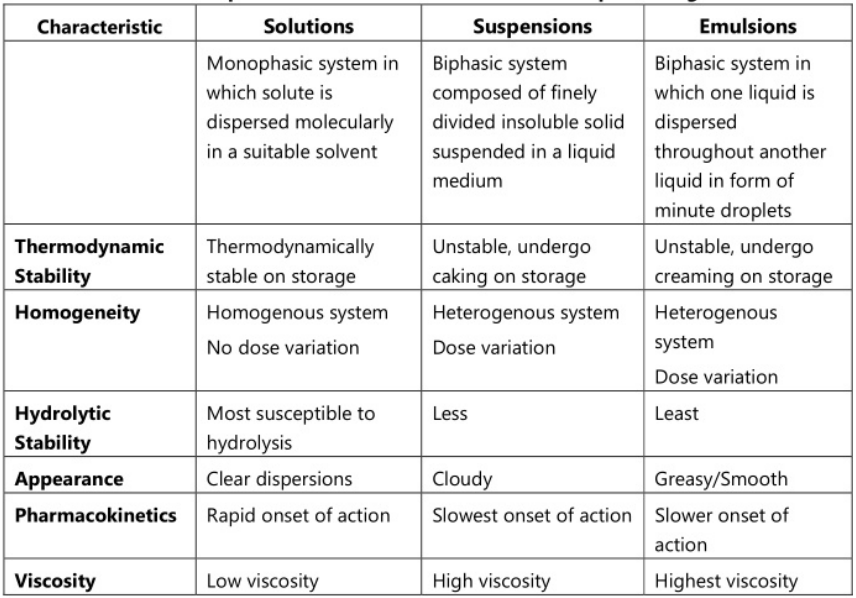
Table of Contents
Advantages of Liquid Dosage Forms
The presentation of drugs as liquid dosage form offers the following advantages:
- The drug is more readily available for absorption from liquid dosage forms as compared to the solid dosage form. By providing the drug in solution, the dissolution phase of the absorption process can be surpassed, providing a faster therapeutic response.
- The doses of drugs can be easily adjusted according to the need of the patient. If the dose of the active ingredient is to be altered, a simple adjustment to the quantity of solution to be taken is all that is required.
- Liquids are easier to swallow than tablets or capsules and are therefore especially suitable for children, the elderly, intensive care, and psychiatric patients.
- Gastric irritation due to certain drugs like potassium chloride and when administered as a solid dosage form is avoided or reduced on administration as a liquid dosage form because of the immediate dilution by gastric content.
- Drugs with large doses can be easily administered as the liquid dosage form.
- The distribution of a drug in liquid dosage forms is better than solid dosage forms.
- Liquid dosage forms are more economical to produce than solid dosage forms.
- Liquid dosage forms can be designed to administer via a number of routes. Parenteral preparations, douches for vaginal use, cutaneous (for use on skin) preparations, and ophthalmic preparations can all be liquids.
Disadvantages of Liquid Dosage Forms
There are also some disadvantages associated with the use of liquid preparations:
- Drugs are usually less stable in liquid dosage forms as compared to solid dosage forms like tablets and capsules, particularly if they are susceptible to hydrolysis,
- Liquids, especially aqueous preparations, are susceptible to microbial contamination.
- Masking the unpleasant taste of a drug in solution is more difficult than when the drug is in a solid dosage form.
- Liquid preparations are usually bulky and therefore inconvenient to store and carry. Liquid dosage forms are always much larger and more bulky than solid formulations. Coupled with this is the fact that pharmaceutical liquids are packed in glass bottles, which are prone to breakage.
- Administration of the correct dose is less precise since it depends on the ability of the patient to measure the correct dose using a suitable measuring device such as a spoon or a dropper.
- The measuring device is to be supplied to the patients for accurate dose administration. This will have cost implications and in addition, counseling is required for its use.
- Suspensions and emulsions have the added drawback that they must be thoroughly shaken to allow accurate dosing.
Excipients used in Formulation of Liquid Dosage Forms
Sweetening agent
Sweeteners are indispensable components of many liquid oral dosage forms, especially those containing bitter or other unacceptable tastes. In fact, sweetening agents may comprise large portions of solid content in most liquid oral dosage forms. Sweeteners are often classified as either nutritive (caloric) or non-nutritive (non-caloric). Non-caloric sweetening agents are preferred for diabetic patients, as ingestion does cause an increase in systemic glucose concentrations. Some of the most commonly used sweeteners include sucrose, sorbitol, mannitol, liquid glucose, honey molasses, saccharin, aspartame, sucralose, and acesulphame-K. The types and concentrations of sweeteners for common prescription liquid medications are reported by Hill, Flaitz, and Frost. Sucrose is the most widely used sweetener, with a long history of use. It is a white crystalline powder, soluble in water and alcohol. It inhibits the growth of microorganisms in solution at sucrose concentrations above 65 wt% by reducing the water-activity coefficient. Official simple syrup is an 85%w/v solution of sucrose in water. During the preparation of sucrose solution, care should be taken to avoid charring and caramelization caused by heat. Sucrose is chemically and physically stable in the pH range of 4.0-8.0. It is frequently used in conjunction with sorbitol, glycerin, and other polyols, which reduces its tendency to crystallize.
One of the manifestations of sucrose crystallization is “cap-locking,” which occurs when sucrose crystallizes on the threads of the bottle cap and interferes with an opening. Liquid glucose is an extremely viscid substance that imparts both body and sweetness to liquid formulations. It is obtained by the incomplete hydrolysis of starch and consists chiefly of dextrose, dextrins, maltose, and water. It imparts a characteristic odor and flavor to the formulation in a similar fashion to honey and molasses, but to a lesser degree. Although liquid glucose is not a pure chemical entity, its method of manufacture can be well controlled, and batch to batch variability is usually not significantly problematic. The same is not true of honey and molasses, in which quality depends on uncontrollable natural factors.
Saccharin (Sweet N Low) is a non-nutritive synthetic sweetening agent. It has approximately 500 times the sweetening power of sucrose, depending on the extent of the strength of the solution. The relative sweetening power is greatest in dilute solutions. Saccharin is a sucrose substitute for diabetics, the obese, and others who do not wish to ingest sucrose. It is commonly found in its sodium salt form, which is more palatable than saccharin and comparatively free of unpleasant after taste. Sodium cyclamate is another synthetic sweetening agent that is approximately 30 times as sweet as sugar. However, its use as an artificial sweetener is banned in the U.S.A. because of the possible toxicity of its metabolite cyclohexylamine. Aspartame is 200 times sweeter than sucrose and, unlike saccharin, has no aftertaste. Its aqueous solubility is adequate for formulation purposes. It is stable in the solid form, but its stability in the solution depends on temperature and pH. It hydrolyzes to aspartyl phenylalanine and diketopiperazine, with a loss in sweetness by aspartame synergistic with saccharin, sucrose, glucose, and cyclamate. In addition, its taste can be improved by adding sodium bicarbonate, gluconate salts, and lactose.
Newer non-caloric sweetening agents have come to market in the last decade. Sucralose (Splenda) is approximately 600 times sweeter than sucrose and differs from sucrose by the substitution of three chlorines for hydroxyl groups. Sucralose is heat stable and stable over a wide pH range affording its utility in formulations prepared at high temperatures. Acesulphame-K is approximately 200 times sweeter than sucrose and is commonly used concomitantly with aspartame to synergistically enhance overall sweetening. This sweetener is also heat stable. Furthermore, Monoammonium glycyrrhizinate has even been used in liquid oral preparations.
Viscosity controlling agents
It is sometimes desirable to increase the viscosity of a liquid, either to serve as an adjunct for palatability or to improve pourability. This can be achieved by increasing the sugar concentration or by incorporating viscosity-controlling agents such as polyvinylpyrrolidone or various cellulosic derivatives (e.g., methylcellulose or sodium carboxymethylcellulose). These compounds form solutions in water that are stable over a wide pH range. Methylcellulose and carboxymethylcellulose are available in a number of different viscosity grades. Carboxymethylcellulose may be used in solutions containing high concentrations of alcohol (up to 50% ) without precipitating. It is precipitated, however, as an insoluble salt of a number of multivalent metal ions such as AT++, Fe+++, and Ca++. Methylcellulose polymers dal does not form insoluble salts with metal ions but can be salted out of solution when the concentration of electrolytes or other dissolved materials exceeds certain limits. These limits may vary from about 2 to 40%, depending on the electrolyte and the type of methylcellulose involved.
Viscosity-inducing polymers should be used with a degree of caution. They are known to form molecular complexes with a variety of organic and inorganic compounds, and in so doing, influence the activity of these compounds. It is conceivable that highly viscid systems that resist dilution by gastrointestinal fluids might impede drug release and absorption.
Buffers
During storage of liquid preparations, degradation of the product, interactions with container components, or dissolution of gases and vapors causes a change in their pH level, which can be prevented by the addition of buffer. A suitable buffer system should have adequate buffer capacity to maintain the pH level of the product. Commonly used buffer systems are phosphates, acetates, citrates, and glutamates. Although buffers ensure pH stability, the buffer system can affect other properties such as solubility and stability. The ionic strength contributions of the buffer systems can affect stability. Buffers can also act adversely as general acid or general base catalysts and cause degradation of the drug substance. Therefore, before selecting any buffer system, the effect of buffer species should be studied.
Antioxidants
Various drugs in the solution are subject to oxidative degradation. Oxidation is defined as a loss of electrons from a compound leading to a change in the oxidation state of the molecule. Such reactions are mediated by free radicals or molecular oxygen and are often catalyzed by metal ions. Moreover, oxidation often involves the addition of oxygen (or other electronegative atoms like halogens) or the removal of hydrogen. Drugs possessing favorable oxidation potential are especially vulnerable to degradation. Agents with an oxidation potential lower than that of the drug in question are called antioxidants. Additionally, certain properties of the selected primary packaging (such as polymer degradation, oxygen transmission rates, impurities, etc.) can readily lead to oxidation of drug molecules in solution and hence may require the addition of antioxidants to maintain product stability. They are added to solutions alone or in combination with a chelating agent or other antioxidants and function by being preferentially oxidized and gradually consumed or by blocking an oxidative chain reaction where they are not consumed.
Salts of sulfites are the most common antioxidants in aqueous solutions and their antioxidant activity depends on their final concentration and the final pH level of the formulation. Generally, sodium metabisulfite is used at low pH, sodium bisulfite at near-neutral pH, and sodium sulfite is used at basic pH. A combination is often used since a single antioxidant may provide incomplete protection. Certain compounds (e.g., citric and ascorbic acids) have been found to act as synergists, increasing the effectiveness of antioxidants, particularly those that block oxidative reactions. Often, chelating agents such as edetic acid derivatives such as ethylenediaminetetraacetate (EDTA) are used in formulations containing trace amounts of heavy metals that would otherwise catalyze oxidative reactions. Moreover, synthetic phenolic compounds, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) serve as hydrogen atom donors and can successfully prevent oxidation of oils and fats in oral liquid formulations.
Flavors
Flavoring can be divided into two major categories: selection and evaluation. Much has been written on both phases of pharmaceutical flavoring, but selection remains a totally empiric activity.
The four basic taste sensations are salty, bitter, sweet, and sour. Some generalizations concerning the selection of flavors to mask specific types of taste have been suggested by Janovsky and by Wesley. (Table 1.2)
| Taste Sanction | Recommended Flavour |
| Salt | Butterscotch, maple, apricot, peach, vanilla, wintergreen mint. |
| Bitter | ild cherry, walnut, chocolate, mint combinations, passion fruit, mint spice, anise. |
| Sweet | Fruit and berry, vanilla |
| Sour | Citrus flavors, licorice, root beer, raspberry. |
A combination of flavoring agents is usually required to mask these taste sensations effectively. Menthol, chloroform, and various salts frequently are used as flavor adjuncts. Menthol and chloroform are sometimes referred to as de-sensitizing agents. They impart a flavor and odor of their own to the product and have a mild anesthetic effect on the sensory receptor organs associated with taste. Monosodium glutamate has been widely used in the food industry, and to a lesser extent, in pharmaceuticals, for its reported ability to enhance natural flavors. A carefully selected panel reported this substance to be effective in reducing the metallic taste of iron-containing liquids, as well as the bitterness and after taste of a variety of other pharmaceutical preparations. It cannot be used in pediatric products.
Chemburkar and Joslin have reported that the partitioning of parabens into flavoring oils from aqueous systems depends on the concentration of the flavoring oil, the nature and concentration of the additives, and pH.
Wesley’s Pharmaceutical Flavor Guide contains suggestions for flavoring over 51 types of pharmaceutical preparations. It and many similar reports provide some guidance for the formulation chemist, but the final selection must result from a trial and error approach. Inherent in this approach is what is referred to as taste fatigue. Repeated samplings of strong-tasting substances soon result in decreased flavor acuity, and therefore, impaired ability to evaluate flavor properly. Preliminary flavoring should be carried out on diluted samples. This is done by preparing flavored vehicles and adding increments of the medicament or other formulation components responsible for the taste problem. The concentration at which the taste of the medicament is perceptible is referred to as the minimum threshold level. The vehicles that are most effective in masking low levels of the drug are candidates for full-strength flavor evaluation.
Flavor evaluation techniques have progressed to a much greater extent than flavor selection. Taste panels can be useful in selecting one of several candidate formulations. This subject, as well as other flavor considerations, has been surveyed in an excellent book assembled by Arthur D. Little, Inc.
Preservative
In recent years, adequate preservation of liquid products has increased in importance. Reports of clinical complications arising from microbial contamination of oral and topical products have originated in several European countries and the United States. Numerous product recalls and tightened regulatory and compendia limits have re-emphasized the need for a die formulator to carefully and thoroughly consider all aspects of the preservative system chosen for a particular formula. In addition to presenting a health hazard to the user, microbial growth can cause marked effects on product stability.
Numerous sources of contamination exist. Including among these are raw materials, processing containers and equipment, the manufacturing environment operators, packaging materials, and the user.
Manufacturing techniques to minimize microbial contamination are presented under the heading “Manufacturing Considerations.” The remainder of this section deals with preservative systems for liquid products.
An ideal preservative can be qualitatively defined as one that meets the following three criteria:
- It must be effective against a broad spectrum of microorganisms.
- It must be physically, chemically, and microbiologically stable for the lifetime of the product.
- It must be non-toxic, non-sensitizing, adequately soluble, compatible with other formulation components, and acceptable with respect to taste and odor at the concentrations used.
No single preservative exists that satisfies all of these requirements for all formulations. The selection of a preservative system must be made on an individual basis, using published information and “in-house” microbiologic studies for guidance. Frequently, a combination of two or more preservatives is needed to achieve the desired antimicrobial effect.
The antimicrobial agents that have been used as preservatives can be classified into four major groupings: acidic, neutral, mercurial, and quaternary ammonium compounds. Table 1.3 lists some representative members of these groupings and the concentration ranges at which they have been used.
| Class | Usual Concentration (%) |
| Acidic | |
| Phenol | 0.2-0.5 |
| Chlorocresol | 0.05-0.1 |
| O-phenyl phenol | 0.005-0.01 |
| Alkyl esters of para hydroxybenzoic acid | 0.001-0.2 |
| Benzoic acid and its salts | 0.1-0.3 |
| Boric acid and its salts | 0.5-1.0 |
| Sorbic acid and its salts | 0.05-0.2 |
| Neutral | |
| Chlorobutanol | 0.5 |
| Benzyl alcohol | 1.0 |
| o-phenylethyl alcohol | 0.2-1.0 |
| Mercurial | |
| Thiomersal | 0.001-0.1 |
| Phenylmercuric acetate and nitrate | 0.002-0.005 |
| Nitromersol | 0.001-0.1 |
| Quaternary Ammonium Compounds | |
| Benzalkonium chloride | 0.004-0.02 |
| Cetylpyridinium chloride | 0.01-0.02 |
The phenols are probably the oldest and best known pharmaceutical preservatives but are little used in oral pharmaceuticals, owing to their characteristic odor and instability when exposed to oxygen. The more useful members of the series, for this application, are the para hydroxy-benzoic acid esters, and the salts of benzoic and sorbic acid. They are adequately soluble in aqueous systems and have been demonstrated to possess both antifungal and antibacterial properties.
Frequently, a combination of two or more esters of para hydroxybenzoic acid is used to achieve the desired antimicrobial effect. Methyl and propyl para hydroxybenzoic acid, for example, are often used together in a ratio of 10 to 1, respectively. The use of more than one ester makes possible a higher total preservative concentration, owing to the independent solubilities of each, and according to some researchers, serves to potentiate the antimicrobial effect. The solubilities of a series of parabens have been studied at four temperatures. The solubilities are expressed in terms of ideal, actual, and excess free energies.
The remaining three classes of preservatives have been widely used in ophthalmic, nasal, and parenteral products, but have been little used in oral liquids. The neutral preservatives are all volatile alcohols, and their volatility introduces odor problems as well as concern for preservative loss on aging. The mercurials and quaternary ammonium compounds are excellent preservatives. They are, however, subject to a variety of incompatibilities, with mercurials being readily reduced to free mercury and the quaternary compounds being inactivated by a variety of anionic substances. The incompatibilities common to these and other preservatives are discussed by Lachman.
Syrups containing approximately 85% sugar resist bacterial growth by virtue of their exosmotic effect on microorganisms. Syrups that contain less than 85% sucrose, but a sufficient concentration of polyol (such as sorbitol, glycerin, propylene glycol, or polyethylene glycol) to have an exosmotic effect on microorganisms, similarly resist bacterial growth. It is possible, however, for surface dilution to take place in a closed container as a result of solvent evaporation followed by condensation, with the condensate flowing back onto the liquid surface. The resulting diluted surface layer makes an excellent medium for bacterial and fungal growth. These products, therefore, should be designed so that even after dilution, they do not support microbial growth. This can be done either by incorporating a sufficient concentration of preservative, so that a diluted sample of the product resists microorganism growth or by including approximately 5 to 10% ethanol in the formulation. The vapor pressure of ethanol is greater than that of water and normally vaporizes to the surface of the liquid and the cap area, preventing, or at least minimizing, the potential for microorganism growth as a result of surface dilution.
An effectively designed preservative system must retain its antimicrobial activity for the shelf-life of the product. To ensure compliance with this precept, the preservative characteristics of the product in its final form (including formulation and package) must be studied as a function of age. The best method of demonstrating preservative characteristics is by microbiologic evaluation.
To determine whether a specific organism is hazardous, one must consider the nature of the product and its dose, the state of health of the user, and clinical reports on the frequency and severity of infections caused by the microorganism.
The FDA distinguishes between organisms that are “always objectionable” and “usually objectionable.” The former designation is based on only two factors: pathogenicity of the organism and site of use. The latter designation is based on an additional determinant, the state of health of the user. The official compendia are continually reevaluating their standards based on the latest FDA data and guidelines.
Specific organisms generally recognized as undesirable in oral liquids include Salmonella species, Escherichia coli, Enterobacter species, Pseudomonas species (commonly P. aeruginosa), proteolytic species of Clostridium and Candida albicans. Some liquid pharmaceuticals (i.e., ophthalmic solutions) must be processed aseptically and rendered sterile.
Chemical analysis for the antimicrobial constituent frequently provides a helpful guide but can be misleading. Molecular interactions involving preservatives and commonly used pharmaceutical adjuvants, such as surfactants and cellulose derivatives, have been observed. For example, it has been shown that Tween 80 interacts to a significant extent with the methyl and propyl esters of para hydroxybenzoic acid and that the preservative surfactant complex is essentially devoid of antibacterial activity. Chemical analysis for the parahydroxybenzoate esters would not differentiate between the unbound substance (microbiologically active) and the bound substance (microbiologically inactive).
Make sure you also check our other amazing Article on : Dipole Moment