Oxygen is one of the prime growth requirements for the sustenance of microorganisms. Some microorganisms cannot grow in the absence of oxygen (obligate aerobes) while some others cannot live if oxygen is present (obligate anaerobes). Oxygen is ubiquitous in the air so special methods are needed to culture anaerobic microorganisms. The principle of anaerobic cultivation of microorganisms is to reduce the O2 content of the culture medium and remove any oxygen already present inside the system or in the medium. A number of procedures are available for reducing the O2 content of cultures; some simple but suitable mainly for less sensitive organisms, others more complex but necessary for the growth of strict anaerobes. The most common and widely used Method of Cultivation of Anaerobes :
Table of Contents
Candle jar method:
The method provides an environment of 3-5% CO2 preferred by some microorganisms. Although this method is an old one, it is still used for culturing some bacteria such as Neisseria gonorrhoeae.
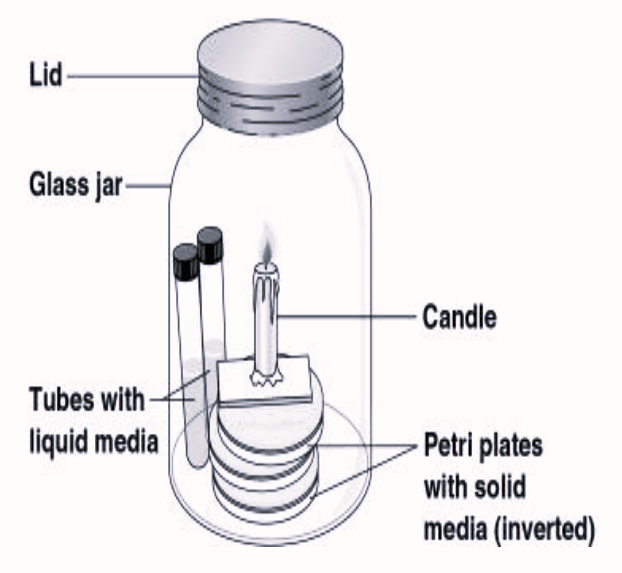
Procedure:
- Place the streaked agar plates of a suspected organism in the center of a glass jar in an inverted position and fix a lighted candle over it. Apply vaseline on the bottom of the bell jar to ascertain that the jar to glass plate seal is airtight. The candle will stop burning due to the consumption of oxygen available and the production of carbon dioxide (Fig 1.1).
- Once the flame quits,3-5% carbon dioxide atmosphere will be created.
- Place the entire assembly in an incubator at 37 °C for 24-48 hours.
Results: The profuse growth of the organism in a plate after incubation reveals that the suspected organism is anaerobic in nature. The presence of aerobic organisms will be indicated by the absence of growth due to the lack of oxygen in such an environment.
Anaerobic jar method:
McIntosh & Filde’s anaerobic jar is the most reliable and widely used method. The method is based on hydrogen generation in the jar which can react with the oxygen available at room temperature catalyzed by palladium pellets to form water. To ensure whether an anaerobic atmosphere exists in the jar, an indicator strip of methylene blue (oxygen indicator) is used that becomes colorless in absence of oxygen. If the strip does not get decolorized within two hours, the gas contains oxygen and a chemical reaction failed to occur (Fig.1.2).
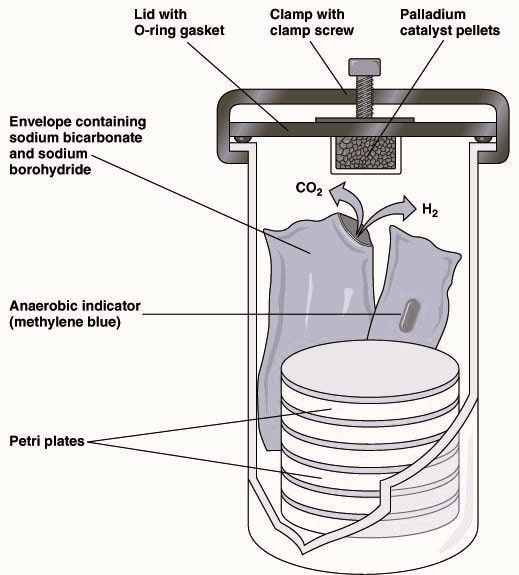
Procedure:
- Keep the streaked plates of a suspected organism from a preferred location in the jar containing gaspak indicator strip of methylene blue and gaspak generator envelopes.
- Pull the strip halfway so that the change in color is visible.
- Cut the envelopes and add 10 ml of the tap water into the open envelope.
- Close the whole assembly and keep it at 37°C of incubation for 24-48 hours.
- Check the jar after 2-3 hours to note if the indicator strip has lost its blue color.
- After 24-48 hours of incubation, remove the lid. If a vacuum holds the inner lid firmly to the jar, break the vacuum by sliding the lid to the edge.
- Examine the plates, followed by staining procedure and biochemical identification of the organism.
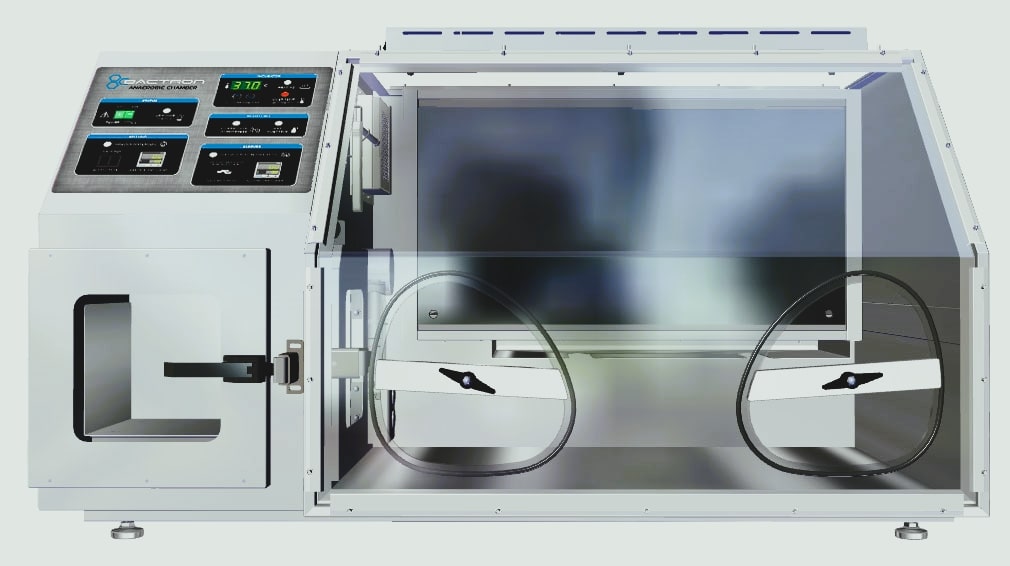
Anaerobic chamber:
This refers to a plastic anaerobic glove box which is a sealed chamber that contains an atmosphere of H2, CO2, and N2. Culture media are placed within the chamber by means of an airlock which can be evacuated and refilled with N2. Any oxygen in the media is slowly removed by reaction with hydrogen, forming water; this reaction is aided by a palladium catalyst. After being rendered oxygen-free, the media are inoculated within the chamber (by means of the glove ports) and incubated (also within the chamber). Fig 1.3
Gas Pak:
It is commercially available as a disposable packet containing pellets of sodium borohydride, cobalt chloride, citric acid, and sodium bicarbonate. Nowadays it is widely used for preparing anaerobic jars. These chemicals generate H2 and CO2 when water is added. H combines with O2 in presence of a catalyst. After inoculated plates are placed inside an air-tight jar, the packet of ‘Gas Pak’ with water added, is kept inside and the lid is tightly closed.
Vacuum and Gas displacement method:
Ideal atmospheric conditions for strict anaerobes can be achieved by displacing the air in a closed container with a mixture of nitrogen and carbon dioxide. A vacuum pump is used to withdraw the air from the chamber containing the culture plates to be incubated. A mixture of the two gasses is then allowed to fill the chamber. The CO2 serves the same purpose as in the Gas Pak system. Many bacteria have difficulty starting growth in the absence of CO2.
Thioglycollate broth method:
This is one of the most common methods of producing an anaerobic environment for microbes by introducing a reducing agent (sodium thioglycollate) into the liquid medium. The reducing agent removes oxygen from the medium through a chemical reaction. Methylene blue or resazurin dye may be added to the medium as indicators. These dyes are colorless in the reduced state (when free oxygen is not present). When the dye molecules become oxidized, their respective colors re-appear (blue for methylene blue and pink resazurin). Resazurin is most often used than methylene blue due to its less inhibitory effects on microorganisms than methylene blue. When allowed to stand, the upper level of the Thioglycollate Broth, where oxygen has diffused into the medium, will have a pink tinge if the broth contains resazurin, or blue if the indicator is methylene blue. The surface of the medium allows the growth of aerobes. Facultative anaerobes thrive throughout the medium. Microaerophiles are localized under the surface while the obligate anaerobes tend to grow throughout the depth of the broth but are more concentrated at the bottom.
Alkaline – pyrogallol method:
The method helps in the isolation of the organism in pure form which is not possible with the broth culture. The reducing agent is placed in the aerial space of the culture tube that allows the anaerobes to grow on an agar slant. An anaerobic condition is established by activating pyrogallol with sodium carbonate, which then acts as a strong reducing agent and removes free oxygen from a tightly stoppered tube.
Procedure:
- Inoculate the nutrient agar slant with any anaerobic microorganism to be tested…
- After inoculation cut off the protruding portion of the cotton plug. With the handle of an inoculating loop, push the remaining half of the plug down into the tube to within 1 cm from the tip of an agar slant. The cotton plug must fit tightly so that the chemicals placed on it will not seep into the agar until the tube is inverted. At the same time, it must allow free passage of gases between the two compartments created by the cotton plug.
- With a clean spoon, add pyrogallol crystals to within about 2 cm of the neck of the tube.
- Add one dropper full of 10 % sodium carbonate (w/vaq. Sol.) to the dry pyrogallol.
- Insert a rubber stopper into the neck of the tube and simultaneously invert the tube and twist the rubber stopper into place until an air-tight seal is formed.
- Once the tube is inverted, keep it in a wire basket in an inverted position.
- Incubate at 35°C for 24-48 hours.
- Note the color change in the medium which may help in the identification of the anaerobe.
Brewer anaerobic culture plate method:
J.H. Brewer introduced a specially designed Petri dish cover to be used with an anaerobic medium for the surface cultivation of anaerobes. The method is convenient for the isolation of colonies which is not possible with Thioglycollate Broth culture and inconvenient by the Pyrogallol method. The cover is provided with a circular ridge that rests on the medium, providing very rare space above the medium. An anaerobic condition in the space results from the presence of reducing agents in the medium. After streaking on the agar surface, the continental Petri dish cover is replaced by the sterile brewer cover. The medium should be deep enough so that the Brewer cover does not rest on the edge of the bottom dish. The weight of the cover will cause the cover ridge to settle somewhat into the medium.
Make sure you also check our other amazing Article on : Growth Of Animal Cells In Culture