- Solvents and vehicles must meet special purity and other standards to assure safety.
- Added substances (buffer, stabilizers preservatives) should be approved for parenteral products.
- Prepared in controlled areas under strict sanitation standards & personnel specially trained, clothed to maintain sanitation.
- Packing hermetically sealed containers of specific & high purity.
- Volume slightly excess of the labeled size to help administration.
- Volume is limited depending on route and type (single or multiple).
- Dry parenteral should be reconstituted fast with ease (lyophilized).
- The finished product must meet sterility standards.
- Must be pyrogen-free.
- No particulate matter should be present.
- Solvents and vehicles must meet special purity & other standards to assure sterility, stability, and safety.
Table of Contents
Vehicles
Vehicles used should be
- Pharmacologically inert, non-toxic, and compatible with blood, maintain solubility of the drug,
- Be physically and chemically stable.
- Does not affect the pH.
- Must be pyrogen-free.
- No particulate matter.
- The finished product must meet sterility standards.
- Water is the ideal vehicle for most injections since aqueous preparations are well tolerated by the body.
- Easy visual inspection for particulate contamination, chemical precipitation, and color change.
- It may accelerate drug hydrolysis resulting in inert or toxic products.
- Solvents and vehicles must meet special purity and other standards to reassure sterility, stability, and safety.
Ideal properties of vehicle used should be
- The vehicle should be pharmacologically inert.
- It should be non-toxic and compatible with blood.
- It should maintain the solubility of the drug (Active Pharmaceutical Ingredient, API).
- It should be physically and chemically stable.
- It does not affect the pH of the dosage form.
- It must be free from pyrogen.
- It should not contain particulate matter.
- The finished product must meet sterility standards.
- Water is the ideal vehicle for most injections since aqueous preparations are well tolerated by the body.
- Easy visual inspection for particulate contamination, chemical precipitation, and color change.
- It may accelerate drug hydrolysis resulting in inert or toxic products.
- The vehicle can be water, water-miscible co-solvents, or non-aqueous solvents.
- Aqueous is preferred.
Non-aqueous may be used due to:
- Drug of limited water solubility.
- Drug susceptible to hydrolysis.
- Desired physicochemical factors (extended-release).
- Intramuscular.
- Must be safe in the amount administered.
- Do not interfere with the therapeutic activity of the drug.
- Selection depends on the formulation.
- Fixed vegetable oils like corn oil, cottonseed oil, peanut oil, sesame oil, castor oil, olive oil.
- Should be properly characterized: purity, iodine number, saponification number.
- Glycerin.
- PEG.
- Propylene glycol.
- Alcohol.
- Less common ethyl oleate, isopropyl myristate, dimethylacetamide.
- Fixed oils should not be administered by IV route and injected only by IM route to produce sustained-release effect. Full information about the Physico-chemical characteristics of the active drug can greatly help in developing a stable and safe parenteral dosage form.
- Crystallinity, polymorphism, particle size, solubility, dissolution, microscopic examination, thermal stability, etc.
- The chemical form of active drug, chemical and/or microbial potency, partition coefficient, spectra, dissociation constant, pH profile (Profile of pH versus solubility and versus stability help in predicting the chemical and physical stability of the drug in solutions or suspension as a result of pH changes due to storage or admixing the drug with infusion fluid.
- Exceptional physical and chemical purity.
- Free of microbes and pyrogen.
- Stable on storage.
- Properly packed and stored to prevent contamination.
- The use of the whole pack during manufacturing.
Preservatives
Preservatives are substances that are incorporated into the parenteral dosage form to maintain stability, ensure sterility and minimize pain and tissue irritation, and aid in parenteral administration.
- Antimicrobial preservatives
- Anti-oxidants
- Buffer
- Chelating Agents / Sequestering Agents
- Cryoprotectants and lyoprotectants
- Inert Gas
- Solubilizing Agents and Surfactants
- Tonicity modifiers
- Viscosity modifiers
- Usually added in multiple-dose vials to protect the product from contamination due to repeated dose withdrawal.
- Also added to most unit-dose parenteral which are not terminally sterilized.
Types of Preservatives
- Acidic: Phenols and parabens (Butyl-P-hydroxybenzoate, Methyl-P-hydroxybenzoate).
- Neutral: Alcohols e.g. Benzyl alcohol
- Mercurial: Thimerosal
- Quaternary ammonium compounds: benzalkonium chloride.
Antioxidants
Antioxidants prevent the oxidation of drugs during sterilization.
Mechanism of action:
- Preferentially oxidized: Ascorbic acid and Na bisulfite, metabisulfite, or sulfite.
- Blocking an oxidation chain reaction: Ascorbic acid, BHT &Tocopherols.
- A complexing agent with a catalyst: EDTA
- Synergistic action: acids (citric, phosphoric, tartaric, ascorbic).
- Added to maintain pH for Solubility, Stability, and Pain reduction.
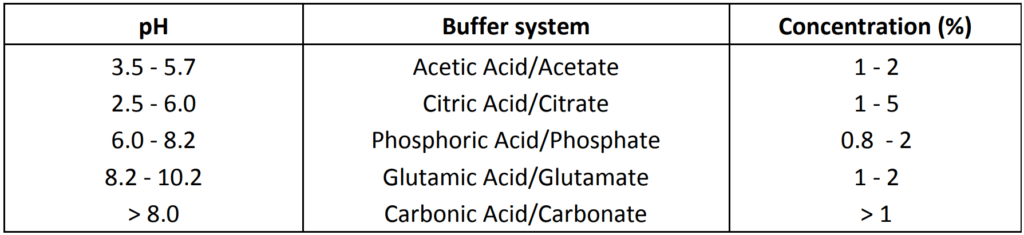
- Other systems used in parenterals: Glycine (pH 6.5 – 7.5), Lactate (pH 3-6), Maleate (pH 2.5 – 5.0), Tartarate (pH 3 – 5)
- Trace quantities of heavy metal ions often catalyze destructive changes in medicaments.
- For example the breakdown of the sulfur-containing rings of benzylpenicillin (copper, lead, mercury, and zinc).
- Oxidation of adrenaline (copper, iron, and chromium).
- Decomposition of oxytetracycline (copper).
- Sources of metal contamination are raw material impurities, a solvent such as H2O, rubber stopper, container, or equipment employed in the manufacturing process.
- The most widely used chelating agents are ethylenediaminetetraacetate (EDTA) derivatives and salts of citric acid and tartaric acids.
Cryoprotectants
- Stabilize and prevent denaturation of proteins from the effect of freezing.
- A hydration shell is maintained around the protein molecules which reduces the denaturing effect of freezing.
- Sugars: Sucrose, Lactose, Glucose, Trehalose
- Polyols: Glycerol, Mannitol, Sorbitol
- Amino Acids: Glycine, Alanine, Lysine
- Polymers: PEG, Dextran, PVP
Lyoprotectants
- The substance which protects drugs especially proteins from degradation during drying (dehydration).
- Sugars: Mannitol, Lactose, Maltose, Maltodextrin, Trehalose, Sucrose
- Amino Acids: Glycine, Histadine, Arginine.
2. In injections in which oxygen is a serious cause of decomposition, improved stability may be obtained by replacing the air in the final container with an inert gas such as nitrogen or carbon dioxide.
3. For the best result, the water must be boiled to remove air and purge the container with the used inert gas, before filling.
Solubilizing Agents and Surfactants
- Surfactants are used extensively in parenteral suspension for wetting powders and provide acceptable syringe ability (e.g. steroids and fat-soluble vitamins).
- Solvents and vehicles must meet special purity and other standards to assure safety.
- Added substances (buffer, stabilizers preservatives) should be approved for parenteral products.
- Prepared in controlled areas under strict sanitation standards and personnel specially trained, clothed to maintain sanitation.
- Packing a hermetically sealed container of specific and high purity.
- Volume slightly excess of the labeled size to help administration.
- Volume is limited depending on route and type (single or multiple).
- Dry parenteral should be reconstituted fast with ease (Lyophilized).
- The finished product must meet sterility standards.
- Must be pyrogen-free.
- No particulate matter.
- Solvents and vehicles must meet special purity and other standards to assure sterility, stability, and safety.
- Natural water: is not used for drinking or any pharmaceutical formulation. It is highly contaminated.
- Drinking water: may be used in the early stage of chemical synthesis and in the early stages of the cleaning of pharmaceutical manufacturing equipment.
- Purified water: (USP monograph), is used in the preparation of some bulk pharmaceutical chemicals, do not use purified water in preparations intended for parenteral administration.
- Must be protected from microbial proliferation.
- Sterile purified water: It is purified water that is packaged and rendered sterile, contains no antimicrobial agent.
- It is not for parenteral administration.
- Water For Injection, USP.
- Most Frequently used for Parenteral Formulations:
> Purified water underwent distillation or reverse osmosis.
> Clear, colorless, odorless, and having a pH of 5 -7.
> Total dissolved solids not more than 1 mg in 100 ml.
> No added substances. – May not be sterile.
> Pyrogen-free.
> Collected in sterile and pyrogen-free containers (glass or glass-lined).
> Must store in a tight container at a suitable temperature.
> Must be used within 24 hours.
- For products to be sterilized after preparation:
> Sterile water for injection with a suitable antimicrobial agent(s)
> Filled in vials/syringe in volume not more than 30 ml.
> The name and concentration of the preservative must be stated. (benzyl alcohol).
> Intended for small volume injectable (multidose vials).
> Not to be used with large volume parenteral (usually with 5mL or less).
> Concern chemical compatibilities. – Suitable vehicle properties.
> No irritation, sensitization, toxicity, or pharmacological activity.
> Should not affect the activity of the drug.
> Should have suitable physicochemical properties for the intended use (stability, viscosity, fluidity with temperature, boiling point, miscible with body fluid, low vapor pressure).
> Purity (eases of purification & standardized). – Must remain clear at 10o C.
> Intramuscular.
> Must be safe in the amount administered.
> Do not interfere with the therapeutic activity of the drug.
> Selection depends on the formulation.
> Fixed vegetable oils.
> Corn oil, cottonseed oil, peanut oil, sesame oil, castor oil, olive oil.
> Should be properly characterized: purity, iodine number, saponification number.
> Glycerin.
> PEG.
> Propylene glycol.
> Alcohol.
> Less common Ethyl oleate, isopropyl myristate, dimethylacetamide.
> Fixed oils must never be administered by IV route and injected only by IM route to produce the sustained-release effect.
- Sterile products are dosage forms of therapeutic agents that are free of viable microorganisms. These include parenteral, ophthalmic, and irrigation preparations.
- Parenterals are unique as they are injected through the skin or mucous membranes into internal body compartments. So, they must be free from microbial contamination and toxic components as well as possess an exceptionally high level of purity.
- Parenteral may be given by various routes: Intravenous, intramuscular, subcutaneous, intradermal, and intraperitoneal.
When given through the intravascular route, complete drug bioavailability occurs immediately. Similarly for other routes at least blood vessel wall or tissue cell wall must be permeated before the drug enters the circulation. Most permeation often occurs by a passive diffusion mechanism. It is favorable when the drug has both lipophilic and hydrophilic properties. Especially lipophilic drugs are being major permeated through passive diffusion. Once in circulating the blood, the physiological effect of the drug is affected by the degree of binding to the plasma proteins and by its rate of elimination by hepatic metabolism and / renal excretion.
Intravenous and intraspinal preparations are most of the time given in the form of aqueous solutions and non-aqueous are rarely given. The danger of blockage of fine capillaries embolism, particularly in the brain precludes the use of form other than solutions for IV administration, although emulsions have been given in which the particle size of the dispersed phase is carefully controlled less than 5 µm. Preparation gave intramuscularly, subcutaneously, or intradermally can be administered as a solution, suspension, or emulsion.
The nature of preparation can influence significantly the rapidity of onset of therapeutic effect from the drug. Then the duration of effect and the form of the absorption pattern are achieved. Therefore the formulation for a parenteral product must be integrated carefully with its intended administration in a patient. The chemical and physical properties of the drug must be determined, its interaction with any desired excipients must be studied and the effect of each step of the process must be studied.
Irrigating solutions: These are also required to meet the same standards as parenteral preparations because, during an irrigating procedure, considerable amounts of these solutions can enter the bloodstream directly through open blood vessels of wounds or abraded mucous membranes.
Effect of Route of Administration:
The intended route of administration has a marked effect on the formulation of a parenteral product. The volume in which a dose of the drug must be encompassed is one factor to consider.
- For intracutaneous injections a volume of more than 0.2 ml rarely is used because tissue volume is small and compact; also, absorption is quite slow owing to the lack of blood vessels.
- Volumes of 1 ml or less may be injected subcutaneously and only.
- Occasionally volumes of more than 2 ml are given intramuscularly.
- Volumes of 10 ml or less may be given intraspinal, but only by the IV route may large volumes be given safely, provided careful control of the rate of administration is undertaken.
Isotonicity (same osmotic pressure) is a characteristic that is probably of the greatest significance for intraspinal injections because the circulation of the cerebrospinal fluid is slow, and disturbances of osmotic pressure quickly cause headaches and vomiting. Isotonicity is preferable for the comfort of the patient but is not essential for SC and IM injections. Generally, IV solution should be isotonic, although slow administration of a paratonic solution may be performed safely if rapid dilution with the blood occurs. Suspension should not be given intravenously because of the danger of the blockage of small blood vessels (embolism). Aqueous or oleaginous suspension cannot be given subcutaneously because of the pain and irritation.
Ophthalmic preparations: These are formulated in much the same way as parenteral solutions. Eye being sensitive to irritation, so formulation should be directed towards minimizing irritation. Suspension of solids has been used in the eye when the therapeutic need superseded the need to avoid irritating effects for example corticosteroids.
Sterile products are mostly
- Frequently solutions or
- Suspensions or
- Solid pellets for tissue implantation.
The control of a process to minimize contamination for a small number of such products can be achieved with relative ease. As the number of products increases, the problems of controlling the process to prevent contamination multiply. Therefore, the preparation of sterile products has become a highly specialized area in pharmaceutical processing. The standards well-known, the attitude of personnel, and the process control must be of a superior level.
Make sure you also check our other amazing Article on : Parenterals