The test is for the measurement of the bactericidal activity of a chemical compound in relation to phenol. This test is carried out by measuring the concentration at which a chemical is equal in effectiveness to phenol. Staphylococcus aureus or Salmonella typhi is used for this test which is inoculated at 20-37°C for 2-3 days. Phenol coefficient is calculated by:
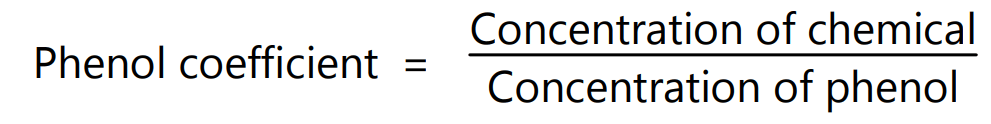
If a chemical is equal in effectiveness to phenol at the same concentration then its phenol coefficient is = 1. If the concentration of the chemical is less than phenol then the phenol coefficient is greater than 1.
Example: Phenol dilution = 1/50 and the maximum effective dilution for disinfectant (x) = 1/ 450.
Then phenol coefficient = 9.
It is determined by two methods:
- Rideal Walker Method
- Chick Martin Test
Table of Contents
Rideal Walker Test (R-W Test)
It is the ratio of the dilution of the disinfectant that kills a microorganism to the dilution of phenol that kills the organism at the same time under identical conditions. The test is based on the principle of dilutions of the test disinfectant is compared with the standard dilutions of the phenol (usually 1 in 95) further activity against Salmonella typhi.
Procedure:
Preparation of sample: The disinfectant fluids sample is mixed thoroughly and is withdrawn from the middle of the sample without forming an air bubble.
Apparatus: A loop, 4 mm in internal diameter is made at end of a 0.376 mm wire of platinum or platinum-iridium alloy, 38 mm long from the loop to the holder. The loop is bent at such an angle to the length of the wire as will facilitate in removal vertically from the surface of the liquid by the loop horizontal.
Incubator: Maintained at 37°C ± 1°C. Pipettes: Standard graduated pipettes of capacity 10 ml, 5 ml, and 1 ml are required.
Dropping Pipette: Made to deliver 0.2 ml. Medication Tubes: 5 sterile plugged rimless test tubes 125 mm × 22 mm made of hard neutral glass.
Broth Tubes: About two dozen of standard measuring cylinders, stoppered and graduated are used.
Standard measuring cylinders stoppered and graduated: 500 ml graduated in 10 ml: one 100 ml graduated 1 ml: five.
Reagents:
Broth: Broth is prepared with the mixture of the following ingredients:
Meat extract (Microbiological grade) 20 g.
Peptone (Microbiological grade) 20 g.
Sodium Chloride (Reagent Quality) 10 g.
Distilled Water: 1000 ml.
Dissolved the solids in distilled water then sodium hydroxide is added to neutralize the solution and boiled to bring down phosphates and filter while hot. pH 7.6 is adjusted with normal hydrochloric acid. The broth is then sterilized by autoclaving at 15 Ibs with pressure for 20 minutes. It is then filtered and placed in 5 ml quantities in sterilized broth tubes and is sterilized by autoclaving at 15 Ibs pressure for 10 minutes.
Test Organism: The test organism Salmonella typhi (NCTC 786) is used. This culture is maintained by a weekly sub-culture on a nutrient agar slope and incubated in the sub-culture for 24 hours at 37°C and then stored in the refrigerator at a temperature below 22°C. For the purpose of the test, a little of the growth from the most recent sub-culture in nutrient agar slope is placed in a tube of R.W. broth and incubated for 23 hours at 38°C. A standard loopful is then transferred to a second tube and incubated. This is done for at least three times before a test is carried out.
Standard Phenol: Phenol of 5 percent w/v solution is prepared at 40.5°C in sterile distilled water. Test dilutions are prepared from this stock solution containing 1 g of phenol in each 95, 100, 105, 155 ml of the solution made.
Sample dilutions: A test portion of 5 ml is withdrawn and discharged into about 480 ml of sterile distilled water in a 500 ml glass stoppered sterile measuring cylinder and the pipette is rinsed three times or more in the clear liquid. The whole is then made up to 500 ml with sterile distilled water. The cylinder is stoppered and the contents are mixed thoroughly. Suitable test dilutions in sterile distilled water are then immediately prepared.
Procedure:
5 ml of four chosen dilutions of the disinfectant are placed in four medication tubes and then placed in a water bath at a temperature between 17°C and 19°C, with the strongest dilution on the left. The fifth medication tube containing 5 ml of the particular phenol dilution is placed on the right. When the content of the medication tubes and broth culture of the test organism has reached the temperature of the water bath, starting at Zero time, 0.2 ml of the culture is added to the left-hand medication tube and the tube is shaken gently. After 30 seconds the next tube is inoculated similarly and the process is repeated with each successive tube at an interval of 30 seconds until the phenol control has been inoculated. A loopful of the well-shaken content of the tube at the extreme left is withdrawn after 30 seconds and placed in a tube containing 5 ml of broth medium. Thirty seconds after this, a similar operation is performed on the second medication tube. The procedure is repeated at an interval of 30 seconds with each of the five medication tubes working from left to right until four sets of cultures have been made i.e. at 2.5, 5, 7.5, and 10 minutes respectively after exposure. Then the inoculated broth tubes are incubated for 48 hours at 37°C and observed for the growth of the test organisms by forming turbidity of the broth.
A typical result and the method of calculating the coefficient are given below.
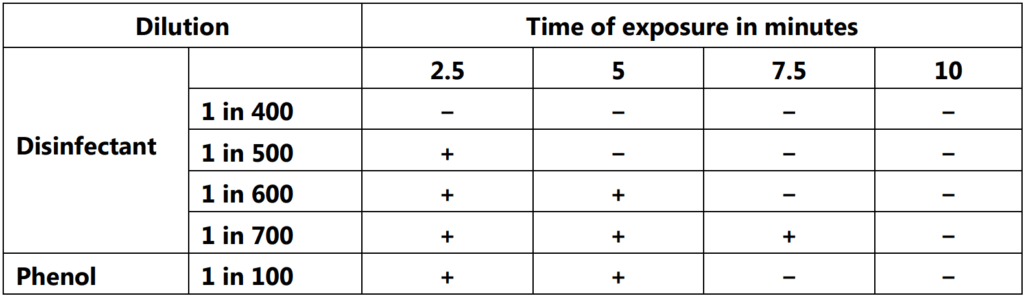
Calculation of the Coefficient :
The Rideal-Walker coefficient is obtained by dividing the dilution of test disinfectant which does not kill all the organisms after 2.5 and 5 minutes but after 7.5 and 10 minutes, by the dilution of phenol that produces a similar effect.
The result indicated that a phenol coefficient greater than 1 means that the disinfectant is better than phenol.
Disadvantages of the Rideal-Walker Test:
- No organic matter is included.
- Microorganism Salmonella typhi may not be appropriate.
- The time allowed for disinfection is short.
- Used to evaluate phenolic typed is infections only.
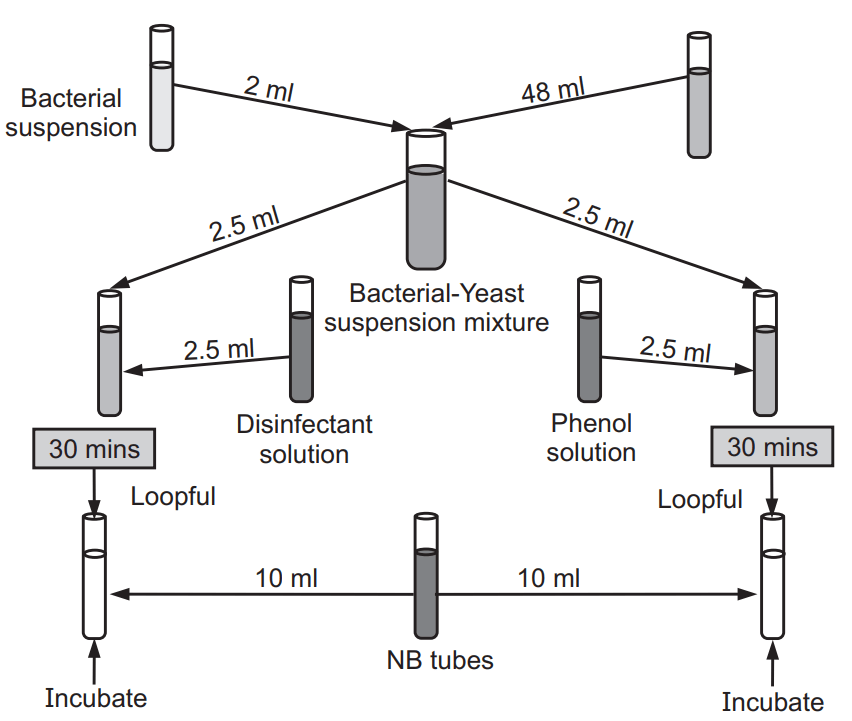
Chick Martin Test
This test is carried out in the presence of organic matter like 3% human feces or dried yeast. This test is a modified method of RW test or qualitative suspension test.
Procedure
- Serial dilution of the test solution and phenol is prepared in distilled water.
- Prepared 5% solution of phenol.
- To this 5% yeast suspension is added after 30 minutes.
- Set up a row of nine test tubes. To each test tube added 2.5 ml of diluted disinfectant at 30 seconds intervals. Allow the reaction to proceed at 20°C (room temperature) in a water bath.
- To this solution, Salmonella typhi is added.
- After a contact time of 30 minutes, the above mixture is transferred to the freshly prepared 10 ml of broth.
- Set up two rows of 10 ml nutrient broth, one for each dilution of disinfectant under test and of the Phenol (i.e. two at each disinfectant and phenol dilution).
- The test tubes are incubated at 37°C for 48 hours.
- Finally, the presence of microorganisms is calculated.
Calculation:

Sample result:
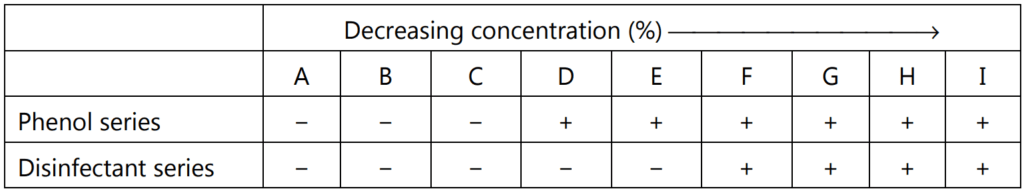
(+) = Growth; (−) = No growth
Average of phenol series = C + D/2
Average of disinfectant series = E + F/2
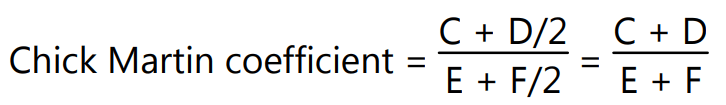
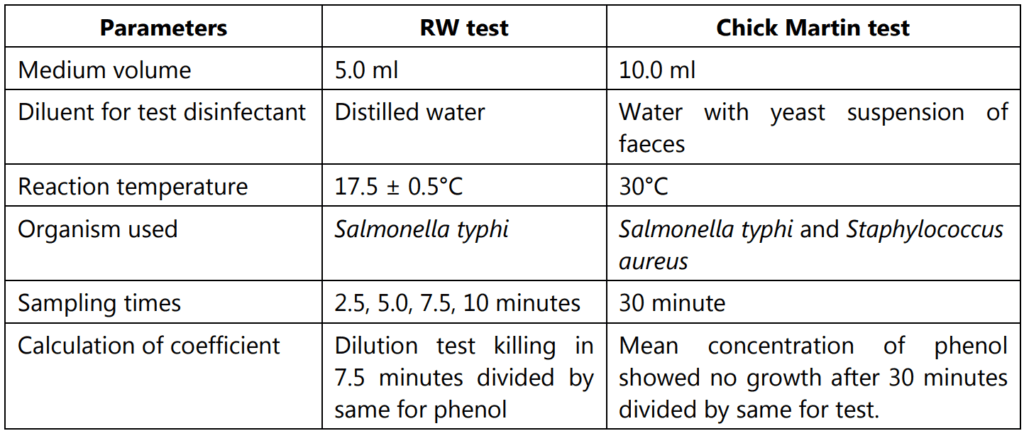
There are some differences between the RW test and Chick Martin test which are tabulated in
Table 1.1
AOAC phenol coefficient test:
AOAC means the American Association of Official Analytical Chemists.
Procedure:
- Dilutions of disinfectant are made from a 1% stock solution.
- From 5% stock solution of phenol, made further dilutions.
- Used 1 : 80 and 1 : 90 for Pseudomonas aeruginosa.
- Used 1: 90 and 1: 100 for Salmonella typhi.
- Used 1: 60 and 1: 70 for Staphylococcus aureus.
- Placed tubes with 5 ml of each final disinfectant dilution and phenol in a water bath at 20°C.
- Placed tubes with a standardized suspension of test organisms in the water bath.
- Added 0.5 ml of test culture to each dilution and phenol.
- Mixed gently and transfer a loopful to neutralizing media at 5, 10, and 15 minutes intervals.
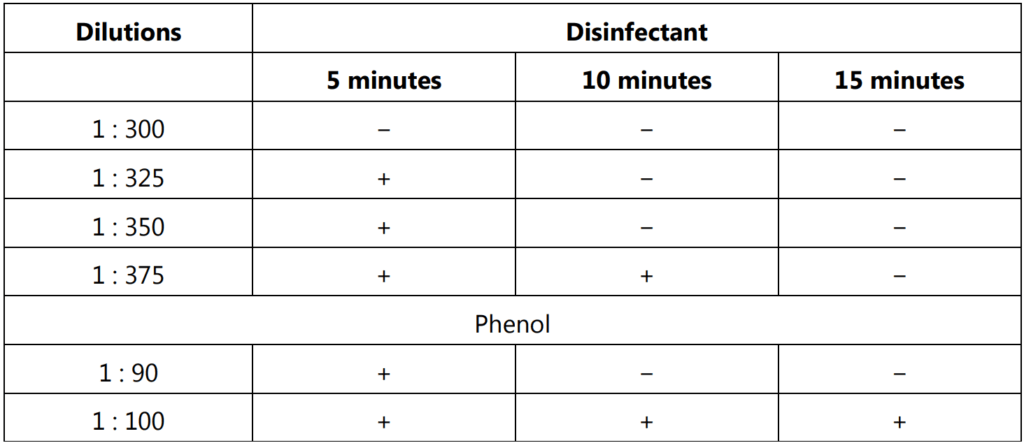
In this method, the Phenol Coefficient is given by the highest dilution of disinfectant killing test organism in 10 minutes but not in 5 minutes divided by the highest dilution of phenol showing the same result. The calculation of the coefficient is tabulated in Table 1.2.
Crown Test: This test is intended for testing white disinfectants in finely dispersed emulsions of coal tar acids or similar acids from petroleum. This test is nearly similar to the Chick-Martin test, but in this test, disinfectant is diluted in sterile artificial seawater, and gelatin and rice starch are included as organic matter.
FDA Phenol Coefficient Test: In this technique, water is used as a diluent for disinfectant. The reaction volume mixture is 10 ml and the medium pH is 6.8. Salmonella typhi is used as a standard organism in this test. The test sample is inoculated in the medium at 5, 10, 15 minutes intervals. The coefficient is calculated by dilution test killing in 10 but not in 5 minutes divided by the same for phenol.
The below table reflected the comparative study between all the biocidal evaluation tests (Table 1.3).
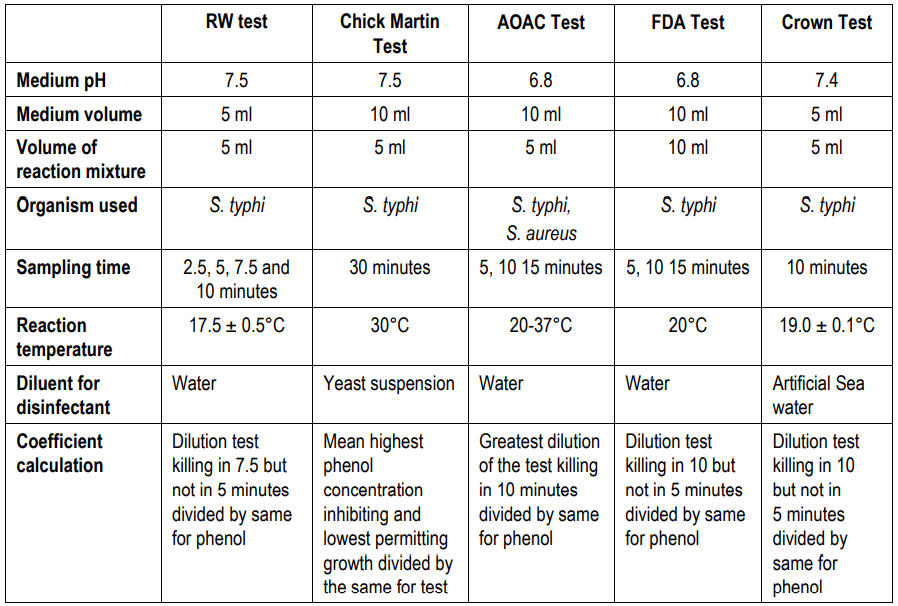
(Phenol coefficient tests)
Counting Method
A mainly viable count method is used which is known as the quantitative suspension test. The count is taken after suitable contact time and the proportion of viable cells are observed. The main advantages of the viable count are
- It counts viable cells only i.e. growing cells.
- It is possible to see cell morphology.
- It can differentiate between different microorganisms.
- This method is used for different types of samples (milk and milk products).
Some of the disadvantages are like:
- The method takes a longer time.
- The method is tedious and requires a lot of test tubes.
Types of Viable Count
The method is two types namely spread plate method and pour plate method. All these methods have three basic steps viz. dilution, Plating, and incubation.
Spread Plate Method:
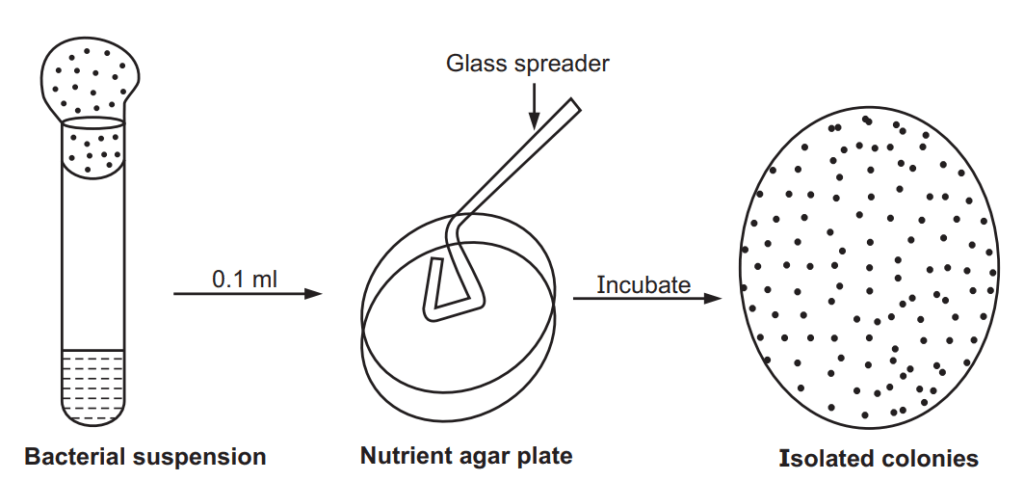
Principle: The method involves using a sterilized spreader with a smooth surface made of metal or glass to apply a small number of bacteria suspended in a solution over a plate. The plate is dried at room temperature to absorb the bacteria in agar very fast. A successful spread plate will have a countable number of isolated bacterial colonies evenly distributed on the plate.
Method (Fig 1.2)
- Dilution series are made from a sample.
- 0.1 ml of sample is pipetted out from the appropriate desired dilution series onto the center of the surface of an agar plate.
- Dipped the L-shaped glass spreader into alcohol and sterile.
- Spread the sample evenly over the surface of the agar using the sterile glass spreader.
- Incubated the plate at 37°C for 24 hours.
- Calculate the Colony Forming Unit (CFU) value of the sample.
- Counted colonies were further multiplied by the dilution factor to determine the number of CFU/mL in the original sample.
Advantages
- With this method, the total number of colony-forming units on a single plate is enumerated.
- It is used to calculate the concentration of cells in the tube from the plated sample.
- The method is used in enrichment, selection, and screening experiments.
Pour Plate Method:
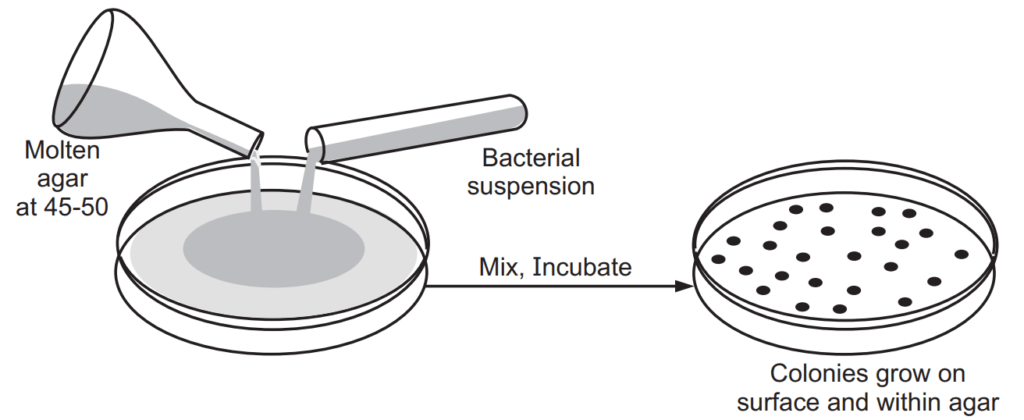
Principle: In this method successive dilution of inoculums is added to molten agar in respective Petri plates and the individual colonies are counted.
Method
- The sample is diluted in sterile distilled water and the agar medium is maintained in the molten stage at 45°C.
- 1 ml of each dilution is added to each sterile petri dish.
- Further added 9 ml of molten agar into the same Petri dishes.
- The contents are mixed properly and allowed to solidify.
- The Petri dishes are incubated at a suitable temperature of 37°C for overnight.
- After incubation, there are different kinds of microbes that are grown as colonies.
- The colonies are then counted.
Advantages:
- The method is simple and useful.
- The method is sensitive.
- It gives the count of viable cells.
Make sure you also check our other amazing Article on : Evaluation of Antimicrobial Agents and Disinfectants

hey this really helped me for my practicals
thanks a Munch