It appears as colourless monoclinic crystals with a faint aromatic odour. Its solution exhibits blue fluorescence. It is composed of phenyl and anthracene, hence the name is phenanthrene. Anthracene is the linear isomer of phenanthrene.
It is used as an adhesive and sealant. Exposure to this compound may cause irritation to the skin and respiratory tract.
Phenanthrene undergoes additional reactions on the double bond between 9 and 10 which showed a strong olefinic character. The 9, 10-bond in phenanthrene closely resembles an alkene double bond in both its length and chemical reactivity. Hence, all chemical reactions of phenanthrene occur across 9, 10-bond.
Chemical Reaction of Phenanthrene
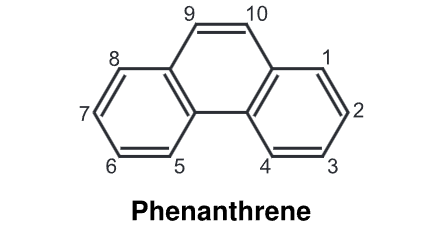
(1) Oxidation: Using, tert-butyl hydroperoxide and molybdenum acetylacetonate [MoO2 (acac)2] it can be oxidized to phenanthrenequinone.
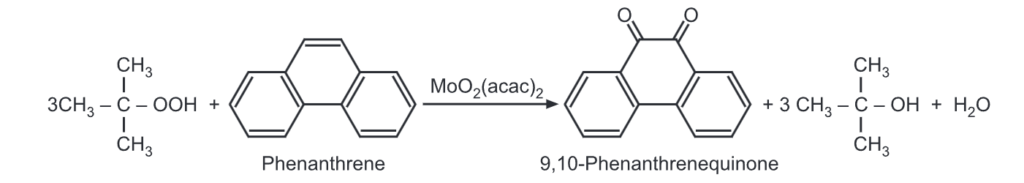
(2) Reduction: When the solid lithium aluminium hydride is vigorously shaken with molten phenanthrene at high temperature, phenanthrene is reduced to 9, 10 dihydrophenanthrene.
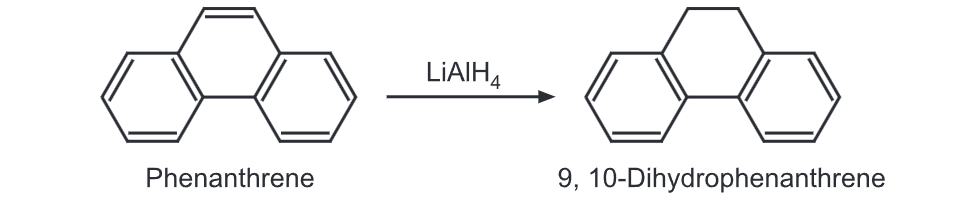
(3) Halogenation: Phenanthrene forms a crystalline dibromide which is sufficiently stable to be isolated. The dibromide can be converted further to mono Bromo product by gentle heating.
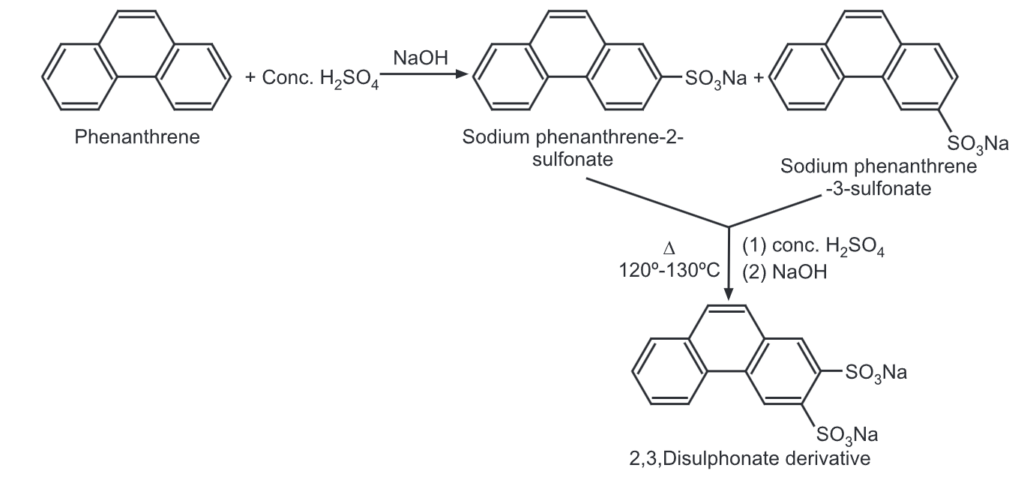
(4) Sulfonation: When phenanthrene is treated with conc. H2SO4, the following two products are obtained after neutralization with NaOH.
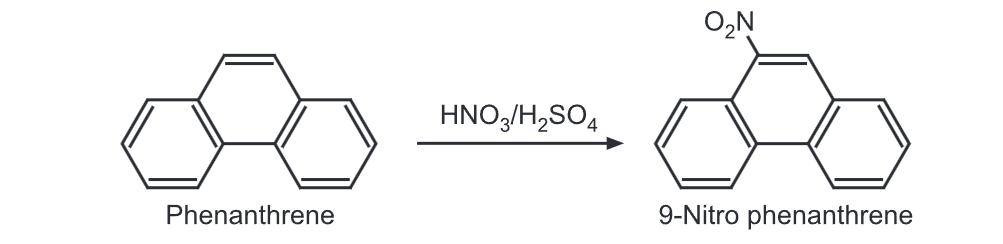
(5) Nitration: Upon treatment with a nitrating mixture, phenanthrene gives 9-nitro derivative.
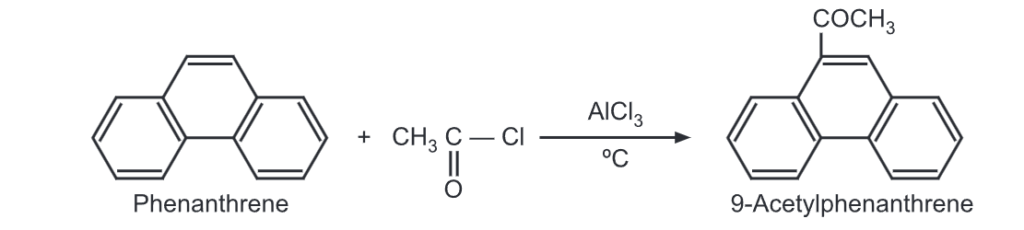
(6) Acylation: Phenanthrene undergoes acylation with acetyl chloride in the presence of aluminium chloride at 0°C to give 9-acetylphenanthrene acetyl.
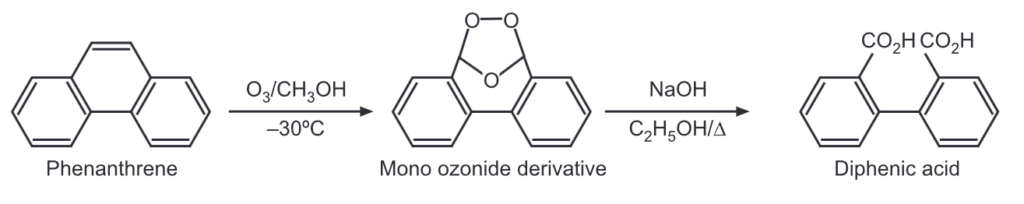
(7) Ozonolysis: Phenanthrene reacts quickly with one mole of ozone at the 9, 10-double bond to give mono ozonide derivative which upon further thermal decomposition, gives 2′-formyl-2-biphenyl carboxylic acid.
Synthesis of Phenanthrene:
(i) Friedel Craft’s acylation:
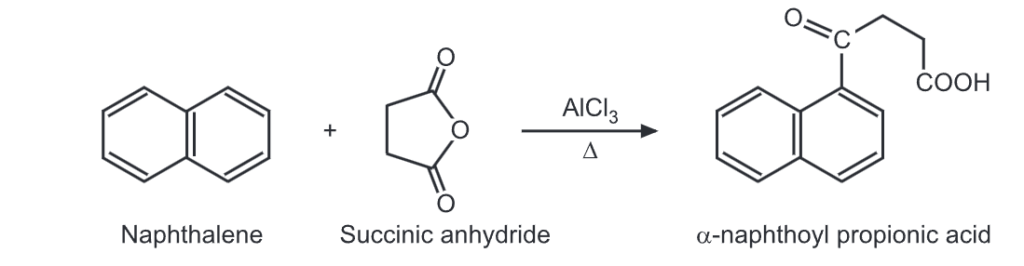
(ii) Clemmensen reduction
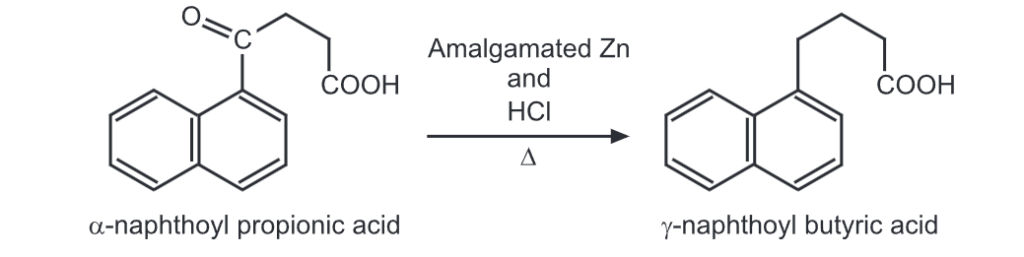
(iii) Ring closure
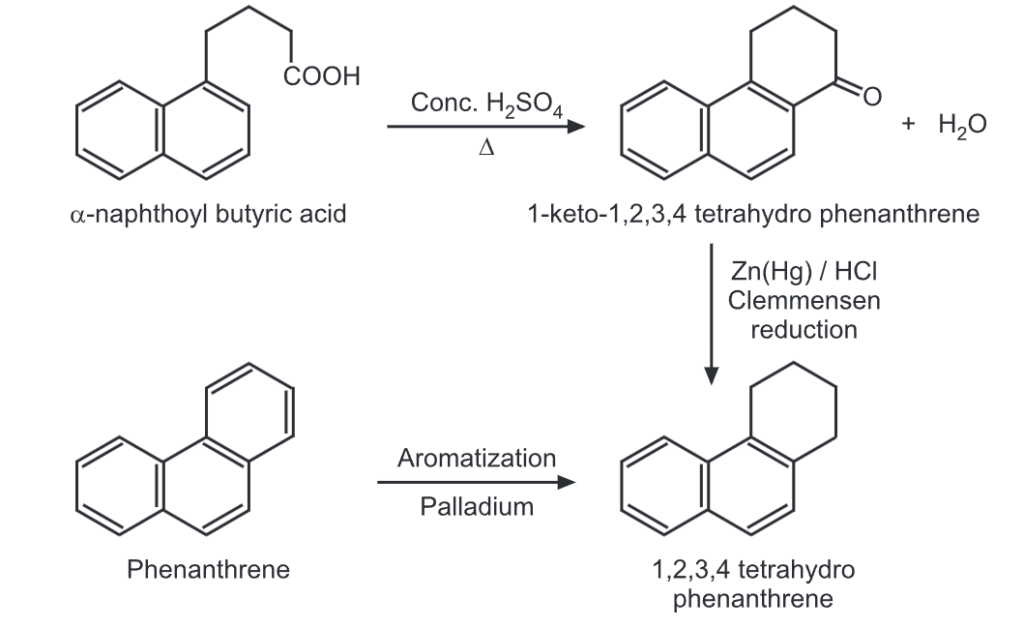
Make sure you also check our other amazing Article on : Chemical Reaction of Naphthalene
