Definition of the Soft gelatin capsules is made of gelatin to which glycerin or a polyhydric alcohol such as sorbitol has been added. Soft gelatin capsules, which contain more moisture than hard capsules, may have a preservative, such as methylparaben and/or propylparaben, to retard microbial growth. These are solid dosage forms in which powder, paste, or liquid medicaments are enclosed in a soft, globular gelatin shell. They may be round, oval, or oblong in shape.
Table of Contents
Nature of Shell and Capsules Contents
Typically, soft gelatin is made up of gelatin, plasticizer, and materials that impart the desired appearance (colorants and/or opacifiers), and sometimes flavors. The formulation of the capsules content for each product is individually developed to fulfill the specification and end-use requirements of the products.
Gelatin: A large number of different shell formulations are available, depending on the nature of the liquid-filled material. Most commonly the gelatin is alkali (base) processed (type B) and it normally constituents 40% of the wet molten gel mass. Type-A acid processed gelatin can be also used.
Plasticizers: It is used to make soft gelatin capsule shell elastic and pliable. The most commonly used plasticizer in soft gelatin is glycerol, although sorbitol and propylene glycol are also used often in combination with glycerol. The amount of plasticizer contributes to the hardness of the final product and may even affect the dissolution and disintegration characteristics, as well as its chemical and physical stability. They are selected on the basis of their compatibility with the fill formulation, ease of processing, and the desired properties of the final products, including hardness, appearance, handling, and physical stability.
Water: It is the most important component of the soft gelatin capsules. It usually accounts for 30 – 40% of the wet gel formulation and its presence is important to ensure proper processing during gel preparation and soft gelatin encapsulation. The levels of water are important for good physical stability because in harsh storage conditions soft gelatin capsules will become either too soft and fuse together, or too hard and embrittled.
Colorants/Opacifiers: Colourants (soluble dyes, or insoluble pigments or lakes) and opacifiers are typically used at low concentrations in the wet formulation. They can be either synthetic or natural and are used to impart the desired shell color for product identification. An opacifier, usually Titanium-dioxide, may be added to produce an opaque shell when the fill formulation is a suspension, or to prevent photo-degradation of light-sensitive fill material. Titanium dioxide is used singly to give a white opaque shell and with combination, it gives a colored opaque shell.
Bloom Strength: Bloom strength is a measurement of cohesive strength of the cross-linking that occurs between gelatin molecules and is proportional to the molecular weight of gelatin. And it is also known as gel strength or gel rigidity of gelatin Bloom Strength: Bloom strength is a measurement of cohesive strength of the cross-linking that occurs between gelatin molecules and is proportional to the molecular weight of gelatin. And it is also known as gel strength or gel rigidity of gelatin.
Viscosity: Viscosity is a measure of the molecular chain length and determines the manufacturing characteristics of the gelatin film. The viscosity for gelatin can be in the range of 25 -45 milipoise.
Flavouring Agent: It may be used in a concentration of not more than 2% are used. Examples are essential oil, ethyl vanillin.
Sweetening Agent: These agents like sugar can be used in a concentration of not more than 5%.
Preservatives: Gelatin is stable when it is dry. Micro-organisms attack gelatin only in presence of moisture. Hence, preservatives are essential. For example, Methylparaben, propylparaben, sodium met bisulfite, potassium bisulfate, etc.
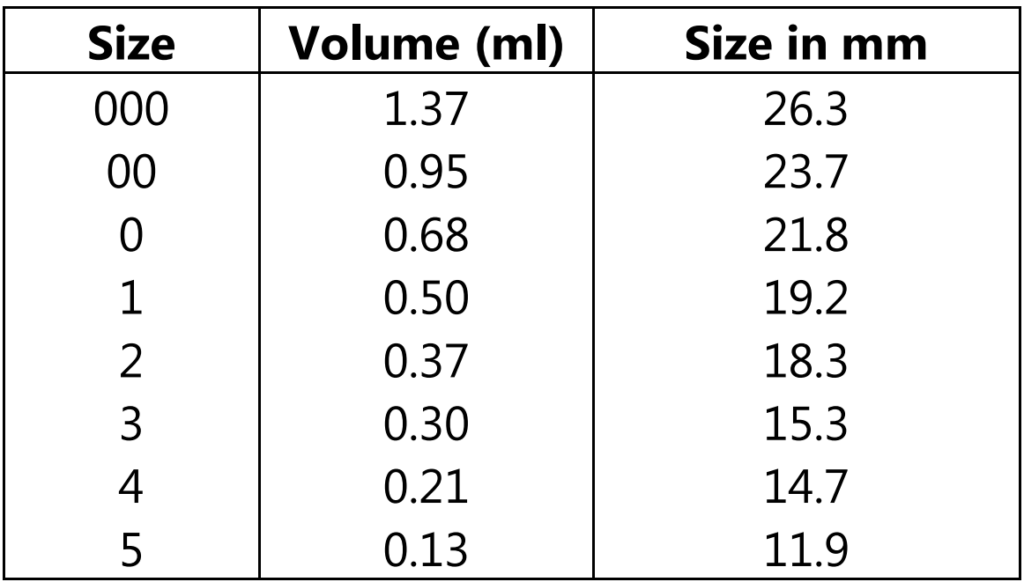
Capsule Size
For Human use, empty capsules ranging in size from 000 the largest to 5 smallest.
Importance of Base Adsorption and Minim/gram
Based adsorption is expressed as the number of grams of liquid base required to produce a capsulate mixture when mixed with one gram of solid. The base adsorption of solid is influenced by such factors as the solid’s particle size and shape, its physical states (fibrous, amorphous, or crystalline), its density, its moisture content, and its oleophilic or hydrophilic nature.
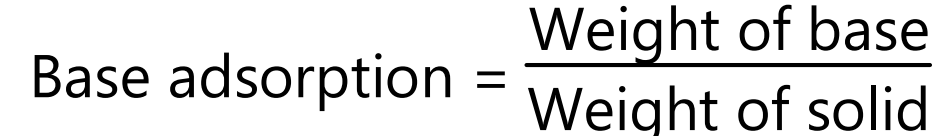
The base adsorption is used to determine the “minim per gram” factor (m/g) of the solid. The minim per gram factor is the volume in minims that is occupied by one gram of the solid plus the weight of liquid base (BA) required to make a capsulate mixture. The minim per gram factor is calculated by dividing the weight of base plus the gram of solid (BA + S) by the weight of mixture (W) per cubic centimeter minims.
Importance
- It helps to determine the base adsorption and fluidity of a mixture.
- It is used to determine the minim per gram factor (M/g) of the solid.
- It is also important of establishing specifications for the control of the physical properties of solid.
- The convenience of using M/g factors is particularly evident in the vitamin field, where there may be many ingredients and numerous combinations.
- They are used to rapidly calculate capsules size.
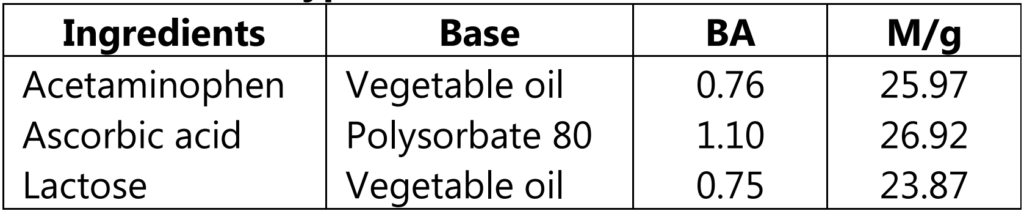
BA and M/g Factors of Some Typical Solids
Production of Soft Gelatin Capsules
Composition of the shell
- The basic component of a soft gelatin shell is gelatin; however, the shell has been plasticized.
- The ratio of dry plasticizer to dry gelatin determines the “hardness” of the shell and can vary from 0.3 – 1.0 for the very hard shell to 1.0 – 1.8 for the very softshell.
- Up to 5% of sugar may be included to give a “chewable” quality to the shell.
- The residual shell moisture content of finished capsules will be in the range of 6-10%.
Formulation
- Formulation of soft gelatin capsules involves liquid, rather than powder technology.
- Material is generally formulated to produce the smallest possible capsules consistent with maximum stability, therapeutic effectiveness, and manufacturing efficiency.
- The liquid is limited to those that do not have an adverse effect on the gelatin shell.
- The pH of liquid can be in the range of 2.5 – 7.5.
Soft Gelatin Capsules are manufactured by following four methods
- Plate process,
- Rotary die process,
- Reciprocating die,
- Accogel machine.
Plate Process:
- Place the gelatin sheet over a die plate containing numerous die pockets.
- Application of vacuum to draw the sheet into the die pockets.
- Fill the pockets with liquid or paste.
- Place another gelatin sheet over the filled pockets, and
- Sandwich under a die press where the capsules are formed and cut out.
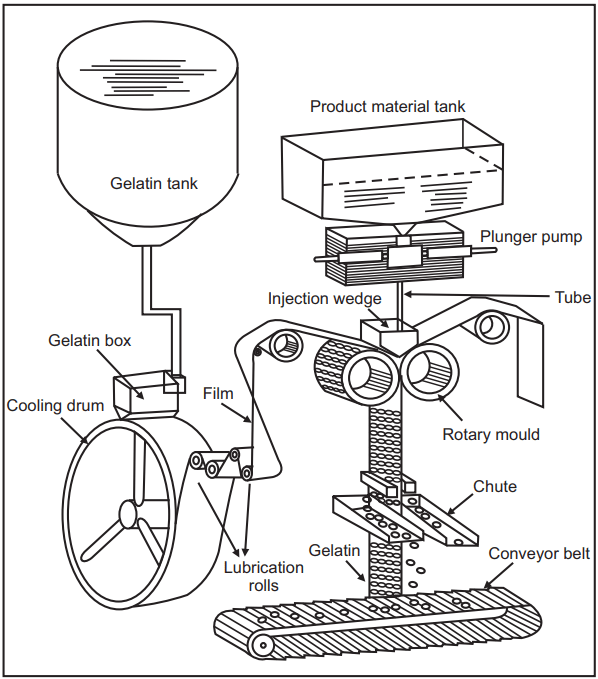
Rotary Die Process:
- In this machine the soft gelatin capsules are prepared and then filled immediately with liquid medicaments, it is having two hoppers and two rotating dies
- The liquid mixture is placed in one hopper and the liquid medicament in another Hopper.
- The two rotating dies rotate in opposite directions when the fluid gelatin mixture enters the machine from the hopper it produces two continuous ribbons.
- These half-shells of the capsule are formed.
- At this stage the measured quantity of the medicament is filled into it with the stroke of a pump with the subsequent movement of them dies, the other half capsule is formed.
- The two halves of the capsules are sealed together by the heat and pressure of the rotating dies.
- As the die rolls rotate, the convergence of the matching die pockets seals and cuts out the filled capsules.
Reciprocating Die Process:
- The early success of the rotary die process led others to develop continuous methods of soft gelatin capsules manufacture.
- One such method, known as the reciprocating die process, was announced in 1949 and was developed by the Nortan Company, Worchester, MA.
Quality Control Tests
Disintegration Test:
The disintegration test for hard and soft gelatin capsules follows the same procedure and uses the same apparatus described in the next chapter for uncoated tablets. The capsules are placed in the basket rack assembly, which is immersed 30 times per minute into a thermostatically controlled fluid at 37°C and observed over the time described in the individual monograph. To satisfy the test, the capsules disintegrate completely into a soft mass having no palpably firm core and only some fragments of the gelatin shell.
Weight variation: The gross weight of 10 intact capsules is determined individually. Then each capsule is cut open and the contents are removed by washing with a suitable solvent. The solvent is allowed to evaporate at room temperature over about 30 minutes, with precautions to avoid uptake or loss of moisture. The individual shells are weighed and the net contents calculated. From the results of the assay directed in the individual monograph, the content of the active ingredient in each of the capsules is determined.
Content Uniformity: Unless otherwise stated in the USP monograph for an individual capsule, the amount of active ingredient, determined by assay, is within the range of 85% to 115% of the label claim for 9 of 10 dosage units assayed, with no unit outside the range of 70% to 125% of the label claim. Additional tests are prescribed when two or three dosage units are outside of the desired range but within the stated extremes.
Dissolution Test:
- The dissolution test is carried out using the dissolution apparatus official in both the U.S.P and IP. • The capsule is placed in a basket, and the basket is immersed in the dissolution medium and caused to rotate at a specified speed.
- The dissolution medium is held in a covered 1000 ml glass vessel and maintained at 370°C ± 0.5°C by means of a constant temperature suitable water bath.
- The stirrer speed and type of dissolution medium are specified in the individual monograph.
Result:
- Six capsules are tested and are accepted if each of them is not less than the monograph specified i.e. +5%.
- If it fails then additional six capsules are tested. The result is accepted if the average of 12 capsules is greater than or equal to p and none of them is less than p − 15%.
- If the capsule still fails the test the additional 12 capsules are tested and are accepted if the average of 24 is greater than the top, if not more than two less than p − 15%, and none of them is less than p − 25%.
Physical Quality Control:
Finally, physical control processing and packaging may be accomplished by the following in continuous operations:
- A capsule diameter sorter allows passing to the next unit of any capsule within ± 0.020 inches of theoretical diameter.
- A capsule color: The capsules are fed to it automatically from the diameter sorter by a pneumatic conveyor. In this unit, any capsule whose color does not conform to the reference color standard for that particular product is discarded others pass the test.
Packaging and Storage of Soft Gelatin Capsules
- Capsules should be packed in well-closed glass or plastic containers and stored in a cool place.
- These types of containers have the advantage over cardboard boxes in that they are more convenient to handle and transport and protect the capsules from moisture and dust.
- To prevent the capsules from rattling a tuft of cotton is placed over and under the capsules in the vials.
- In vials containing very hygroscopic capsules, a packet-containing desiccant like silica gel or anhydrous calcium chloride may be placed to prevent the absorption of excessive moisture by the capsules. Nowadays capsules are strip packaged which provide sanitary handling of medicines, ease in counting and identification.
- Plastic bottle with screw cap (most popular package in the USA).
- Clamshell blister (one-piece plastic that folds over and locks itself; no heating required.
- Blister pack (heat-sealed blister on a cardboard).
- Plastic pail/bucket (economical bulk package).
- Plastic pouch zip-locked (for sale via retail stores or route trucks must be packed in outer case for shipping).
Stability Testing of Soft Gelatin Capsules
Moisture Permeation Test:
The USP requires determination of the moisture permeation characteristics of single-unit and unit-dose containers to ensure their suitability for packaging capsules. The degree and rate of moisture penetration are determined by packaging the dosage unit together with a color-revealing desiccant pellet, exposing the packaged unit to known relative humidity over a specified time interval, observing the desiccant pellet for color change (indicating the absorption of moisture), and comparing the pretest and posttest weight of the packaged unit.
Physical Stability: Unprotected soft gelatin capsules rapidly reach equilibrium with the atmospheric conditions under which they are stored.
- This inherent characteristic warrants a brief discussion of the effects of temperature and humidity on the products.
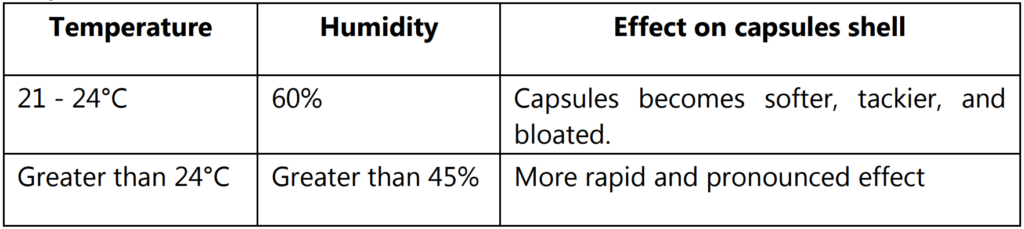
- General statements relative to the effects of temperature and humidity on soft gelatin capsules must be confined to a control capsule that contains mineral oil with a gelatin shell having a dry glycerin to dry gelatin ratio of 0.5 – 1 and water to dry gelatin ratio of 1 – 1 and that is dried to equilibrium with 20 – 30% RH and 21 – 24°C
- The physical stability of soft gelatin capsules is associated primarily with the pickup or loss of water by the capsule shell.
- If these are prevented by proper packaging, the above-controlled capsule should have satisfactory physical stability at a temperature ranging from just above freezing to as high as 600°C.
- As the humidity increases the moisture content pickup of capsules increases. For example: At 30% RH at room temperature shows that gelatin retains about 12% (48 mg) of water and glycerin 7% (14 mg) of water at 60% RH the moisture content should be 17.4%. High humidity (> 60% RH at 21 – 240°C) produce more lasting effects on the capsule shell. The capsule manufacturer routinely conducts accelerated stability tests on new products as an integral part of the product development program.
- The successful results are obtained by conducting at test conditions like
(a) 80% RH at room temperature in an open container
(b) 400°C in an open container
(c) 400°C in a closed container.
- Prior to testing, the capsule should be equilibrated to known humidity at many conditions, preferably 20 – 30% RH at 21 – 24°C.
- Evaluation of the results of the previously described heat test should be made only after the capsules have returned to equilibrium to room temperature.
Stability: 20 to 30 % RH at 21 to 24°C.
Applications of Soft Gelatin Tablets
- As an oral dosage form.
- As a suppository dosage form.
- As a specialty package in tube form, for human and veterinary use, single-dose application for topical, ophthalmic, and rectal ointments.
- It is used in water-immiscible, volatile, and non-volatile liquids such as vegetable and aromatic oils, aromatic and aliphatic hydrocarbons, ether, esters, alcohol, and organic acids.
- Solid is also encapsulated into soft gelatin capsules as the solution in one of the suitable liquid solvents, as suspension, or as dry powder, granules, or pelletized materials.
Make sure you also check our other amazing Article on : Hard gelatin capsules
