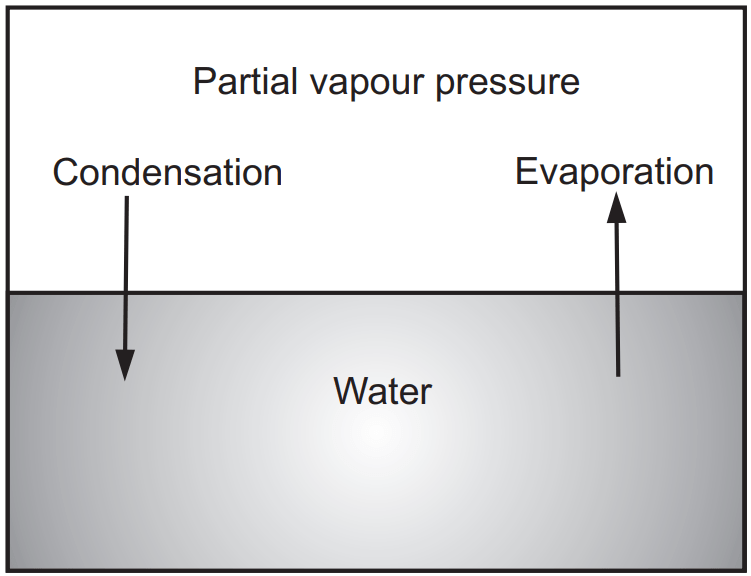
The physical properties of liquids are controlled by the strength and nature of intermolecular attractive forces. The most important properties are vapour pressure, viscosity, surface tension and light absorption and refraction. A liquid placed in a container partially evaporates to establish a pressure of vapour above the liquid. The established pressure depends on the nature of the liquid, and at equilibrium, it becomes constant at any given temperature. This constant vapour pressure is the saturated vapour pressure of the liquid at that temperature. Until the vapour pressure is maintained, no further evaporation observes. As shown in Fig. 1.1, at lower pressures a liquid evaporates into the vapour phase while at higher pressure the vapour tends to condensate till equilibrium establishes. During vaporization, heat is absorbed by the liquid. At any given temperature, the amount of heat required per gram of liquid is a definite quantity called the heat of vaporization of liquid (∆Hv). It is a difference in enthalpies of vapour (Hv) and liquid (Hl), respectively. Therefore,
∆Hv = Hv – Hl……….(1)
During evaporation, ∆Hv is always positive while during condensation it becomes always negative. As per the definition of change of enthalpy, ∆Hv is the difference in internal energy of vapour and liquid
∆Hv = ∆Ev + P ∆Vv……….(2)
where P is vapour pressure and ∆Hv is changed in volume during vapour to liquid transition.
in Temperature
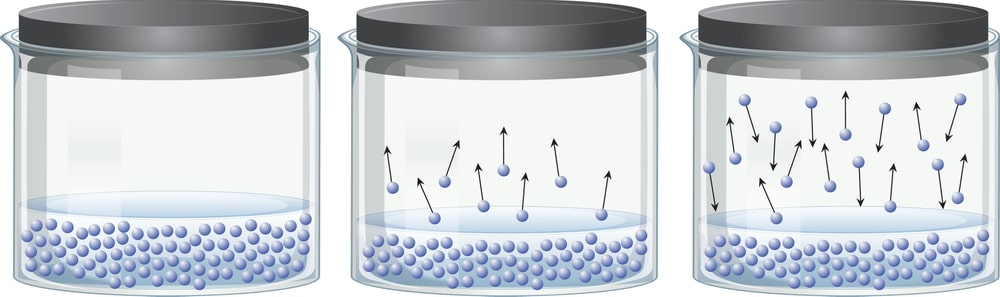
The temperature of a substance depends on the average kinetic energy of its molecules. Average kinetic energy is considered because there is an enormous range of kinetic energies for these molecules. Even at temperatures well below the boiling point of a liquid, some of the particles are moving fast enough to escape from the liquid. During this process the average kinetic energy of the liquid decreases. As a result, the liquid becomes cooler. It, therefore, absorbs energy from its surroundings until it returns to thermal equilibrium. But as soon as this happens, some of the water molecules once again have enough energy to escape from the liquid.
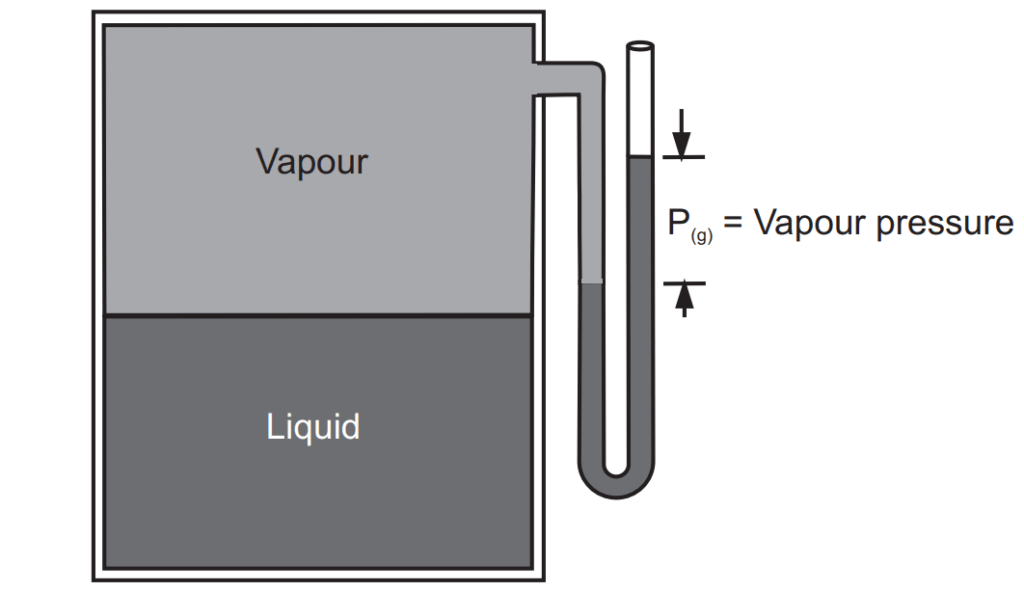
In an open container, this process continues until all the water evaporates. In a closed container, some of the molecules escape from the surface of the liquid to form a vapour. Eventually, the rate at which the liquid evaporates to form a gas becomes equal to the rate at which the vapour condenses to form the liquid. At this point, the system is said to be in equilibrium. As shown in Fig. 1.2, the space above the liquid is saturated with water vapour, and no more water evaporates. The pressure of the water vapour in a closed container at equilibrium is called the vapour pressure.
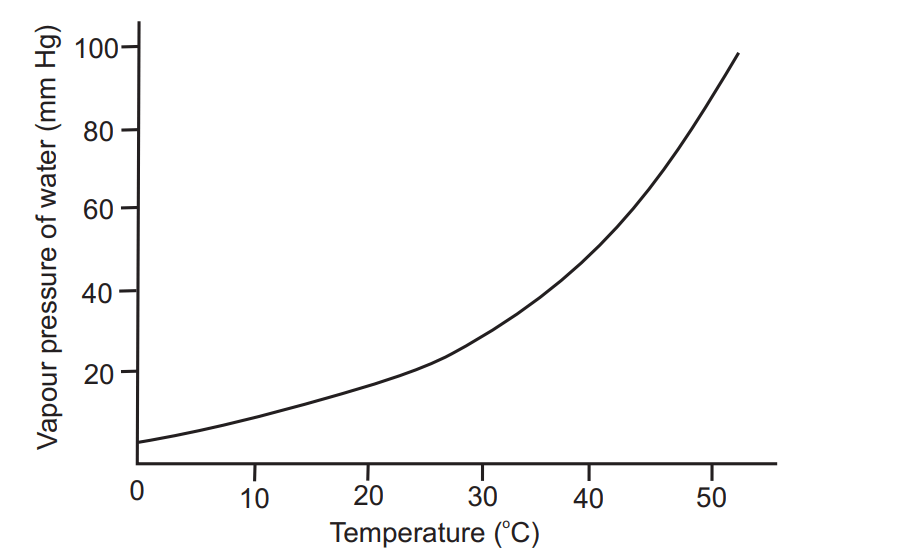
Fig. 1.3 shows that the relationship between vapour pressure and temperature is not linear. The vapour pressure of water increases more rapidly than the temperature of the system.
Table of Contents
Measurement of Vapour Pressure
Vapour pressures of liquids are measured by static and dynamic methods.
Static Method
Vapour pressure of the liquid is generally measured by the isoteniscopic method, which is precise, flexible and convenient over a range of temperatures. A simple apparatus is shown in Fig. 1.4. It consists essentially of an isoteniscopic bulb of 2 cm diameter.
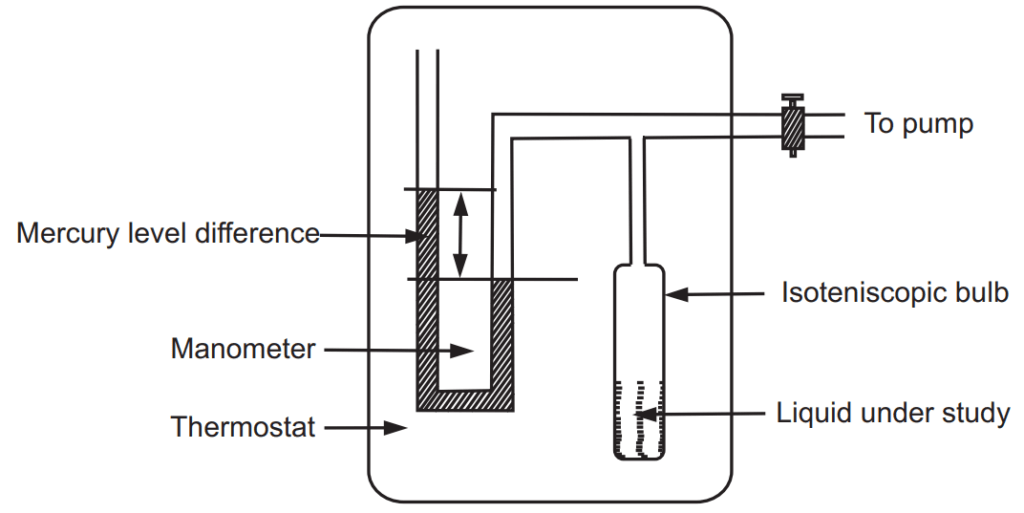
A liquid under test is filled in bulb-up to half level mark, which is connected to mercury manometer and a pump. The air inside the bulb is removed by the application of a vacuum. Now there is no air present in the bulb. To maintain equilibrium, part of the liquid evaporates. The system is maintained at a constant temperature so that the equilibrium between liquid and vapour is attained. The generated vapours exert pressure on mercury present in the column. The difference in height of mercury in the column is determined which is equal to the vapour pressure of that liquid. By maintaining the system at any other temperature, it is possible to determine vapour pressure at that temperature. This method is used for liquids having vapour pressures on higher sides close to one atmosphere.
Dynamic Method
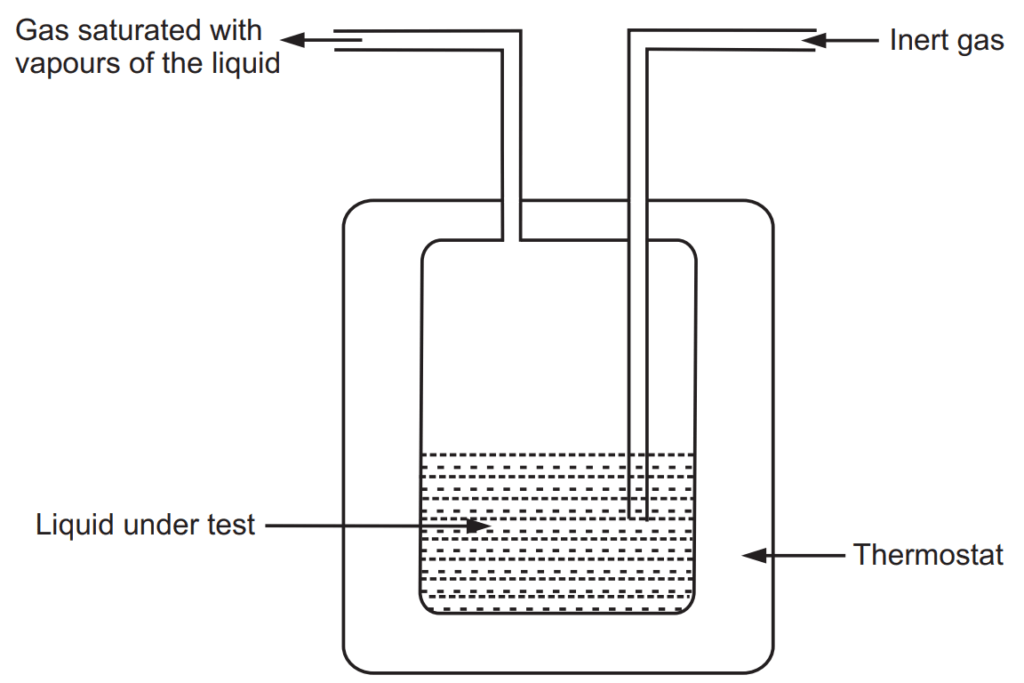
This method is proposed by Walker and is useful, especially in determinations of the very low vapour pressure of liquid mixtures. Great care is required to obtain excellent results. An illustrative apparatus is shown in Fig. 1.5. An inert gas such as nitrogen is passed through the given liquid at a constant temperature. The inert gas is saturated with the vapours of liquid under test and leaves the flask at the exit of the tube. If P is total vapour pressure in the apparatus at saturation, n is the moles of gas passed through and nv is the number of moles of vapour collected. The nv is given as
nv= Wv/Mv……..(3)
where Wv is a loss in weight of liquid and Mv is the molecular weight of liquid. The partial pressure of vapour, P’ is the same as the vapour pressure of the liquid at saturation and can be given as
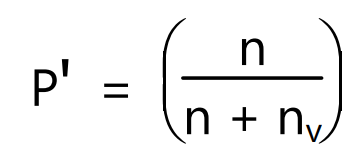
In the other form equation (4) can be written as
P= m/MV × RT……(5)
where m is a loss in weight of liquid as vapour, V is the volume of gas passed through, M is the molecular weight of liquid and R is gas constant.
Boiling Point of Liquids
The boiling point is the temperature at which the vapour pressure of liquid equals the 760 mmHg pressure. However, the increasing temperature can boil liquids at any temperature from its freezing point to critical temperature either or decreasing applied external pressure. Hence, the boiling point of a liquid is the temperature at which the vapour pressure of the liquid is equal to the pressure acting on its surface. Boiling is characterized by the formation of bubbles within it and release from the surface. The change in boiling point with pressure is calculated if the molar heat of vaporization (∆Hv) for the liquid is known. If T1 is the boiling point at pressure P1 and T2 is the boiling point at pressure T2 then using equation (5) boiling point of the liquid at a given pressure is obtained.
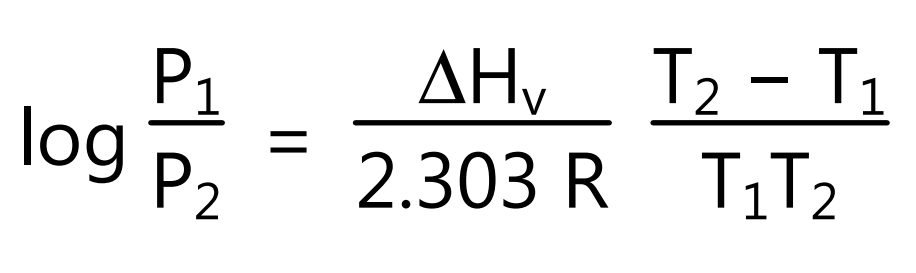
However, when ∆Hv is not known its value is estimated from Trouton’s rule which states that
∆Hv/ Tb = Constant…….(7)
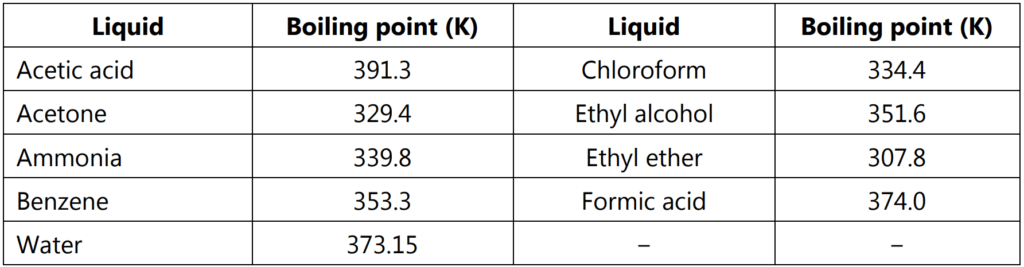
where Tb is the normal boiling point of liquid on the absolute temperature scale. Boiling points of some liquids at one atmospheric pressure are given in Table 1.1.
Boiling Point and Vapour Pressure
Bubbles are formed on heating liquid, which rises to the liquid surface and bursts. When liquid vaporizes, the molecule in vapour state remains together as tiny bubbles with the vapour pressure within it. This vapour pressure in bubbles within the liquids is different from that of atmospheric pressure. When a bubble rises to the surface, it burst to have equal vapour pressure that of the atmosphere. Therefore, the boiling point of a liquid is a temperature at which the vapour pressure of the liquid is equal to atmospheric pressure. Reducing external pressure can reduce the boiling point and at low temperature, it is equal to external pressure. Similarly, increasing external pressure increases the boiling point of liquid and at high temperatures, it is equal to external pressure.
Make sure you also check our other amazing Article on : Parenteral Preparation